Table of Contents
- Arsenic Removal using Lime Precipitation
- Ferrous & Ferric Solutions Precipitation to Remove Arsenic
- Precipitation as a Mixed Calcium Phosphate-Arsenate
- Remove Arsenic by Precipitation as Barium Arsenate
- Precipitation of Titanium (IV) – Arsenic (V) Compounds
- Arsenic Precipitation of MgNH3AsO4.6H2O
- Precipitation as Arsenic Sulphide
- How to Remove Arsenic from Drinking Water
The removal of arsenic from water has been practiced for many years (8-11) but with the recent emphasis on clean water standards there has been renewed activity in relation to the problem. The methods which have been investigated include precipitation, co-precipitation, adsorption, ion exchange and liquid-liquid extraction but to date only precipitation and co-precipitation processes have been adopted for large scale operations.
Precipitation methods have relied on the supposed insolubility of certain arsenates, arsenites and sulphides. The most common treatment process has been to oxidise arsenic to the pentavalent state and precipitate with lime (12), but recent work (13, 14) has shown this to be ineffective. Precipitation of both ferrous and ferric arsenate, aluminium arsenate, titanium arsenate, barium arsenate and mixed calcium arsenate/phosphate has been reported in the literature and all of these methods have been evaluated in the author’s laboratory together with others of likely application to gold processing. The following sections make comment on some of the precipitation and coprecipitation techniques.
Arsenic Removal using Lime Precipitation
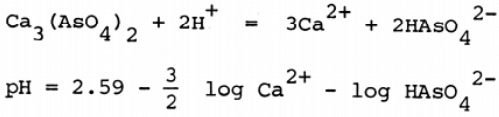
The use of lime to precipitate arsenic (V), supposedly as Ca3(AsO4)2 is based on the work of Chukhlansev (15) . The solubility product for this compound in recent sources of data (16) and a wide acceptance of the insolubility of calcium arsenate is based on Chukhlansev’s results. The chemistry of the lime addition method for gold processing wastes was discussed by Laguitton (12) but in the light of recent work (14) should be revised extensively. Nishimura and Tozowa (17) investigated the solubility of calcium arsenate over a wide range of pH and showed a minimum solubility at about pH = 8 which was significantly higher than had been widely accepted. An interpretation of Nishimura and Tozawa’s results was presented by Robins (13) who proposed that at pH > 8.3 calcium arsenate is unstable and is converted to calcium carbonate by the carbon dioxide in the atmosphere (338 ppm). Robins and Tozawa (14) then pointed out the potential ineffectiveness of the lime process for treating gold processing wastes. Nishimura, Robins and Tozawa (18) have since reported an experimental investigation of the calcium – arsenic – water (air) system, showing the existence of two calcium arsenic (111) compounds and five calcium arsenic (V) compounds. The solubility of the arsenites is significantly greater than the arsenates but all compounds decompose to calcium carbonate above pH = 8.3. The stability region for calcium arsenate exhibits a minimum solubility of about 750 mg/l As(V) for equal concentrations of calcium and arsenic at pH = 8.3. The solubility of Ca3(AsO4)2 which is stable in this region of pH is given by the equations:
The work by Nishimura, Robins and Tozawa would indicate that arsenic removal with lime is effective short term up to a pH > 12 where it is possible to remove arsenic to less than 1 mg/l but the precipitates of Ca3(AsO4) and Ca3(AsO4)2.H2O which are formed in this pH range will decompose to CaCO3 in the natural environment. In a typical tailings pond the rate of decomposition would be controlled by a carbon dioxide transfer rate of about 10 mol/m² year.
Ferrous & Ferric Solutions Precipitation to Remove Arsenic
Shabunin, Gutman and Kogan (8) and Rosehart Lee and Pattyson (19) report the use of both iron (11) and iron (111) solutions to precipitate insoluble arsenates. Similar work in the author’s laboratory indicates that solubilities that can be predicted from stability diagrams (20) are extremely accurate. A minimum solubility for FeAsO4 is about 75 mg/l at pH = 2.2 above which pH decomposition to Fe(OH)3 occurs, but for Fe3(AsO4)2 the minimum solubility is less than 10 mg/l at pH = 6 – 7.
Ferrous arsenate, however, is very quickly oxidised by air from a white precipitate initially to green coloured mixed oxidation state iron arsenate.
Acidic solutions containing arsenic (V) to which ferrous sulphate has been added can then be neutralised with lime and aerated for long periods to form an olive green coloured iron (11) – iron (111) – calcium arsenate solid. This solid is oxidised at extremely slow rates and appears to have an arsenic solubility of about 1 mg/l in the range pH 7 – 8.
Mineralogy literature refers to a number of iron (111) – calcium (11) – arsenates which were thought to be appropriate for residues stabilisation. The minerals arseniosiderite Ca3Fe(AsO4) .3Fe(OH)3, dussertite Ca3(AsO4)2. 3Fe(OH)3, mazapilite Ca3Fe2(AsO4).2FeOOH.5H2O, and yukonite Ca3Fe2(AsO4)2.2Fe(OH)3.5H2O are those most commonly encountered and a study of this system was attempted in the laboratory. It was shown that a number of Fe(111)-Ca(11)-A(V) compounds could be obtained at pH < 4.5 but at higher pH Fe(OH)3 was the stable compound. The solubilities of these compounds was only slightly less than for ferric arsenate so it was concluded that this system is inappropriate for the disposal situation.
Precipitation as a Mixed Calcium Phosphate-Arsenate
A modification of the lime addition process incorporates phosphate into the effluent so that a mixed phosphate-arsenate is precipitated on the addition of lime. Nikolaev and Mazurova (21) proposed a flowsheet for this process in 1972 and FMC Corporation, Philadelphia, were assigned a patent (22) in May 1980 for what appears to be a similar process. The FMC patent claims the application of this process in the range pH 7.0 – 11.5.
The calcium phosphate – water system is complex (23) and although extensively investigated there are some uncertainties in regard to thermo¬dynamic stability and more so in relation to the kinetics of conversion between compounds in aqueous media.
In this laboratory we have found that on adding lime to phosphoric acid solutions the compounds CaHPO4.2H2O, Ca8H2.(PO4)6.5H2O, Ca3(PO4)2.nH2O, and Ca10(PO4)6(OH)2 are formed and interconverted under various conditions but with Ca10(PO4)6(OH)2 hydroxyapatite, the finally stable material (sometimes taking some months to appear).
When arsenic acid – phosphoric acid mixtures with As to P ratio up to 1.0 are precipitated with lime similar reactions occur with the structure of the solid phase being controlled by the phosphate and being almost identical to one of the calcium phosphates. Arsenic solubilities for these compounds is much lower than for the corresponding calcium arsenates, being below 1 mg/l in the range pH = 8.2 – 9.8 in the presence of atmospheric carbon dioxide. At higher pH the compounds will decompose to calcium carbonate which has serious implications to long term storage stability.
One problem with some of the calcium arsenophosphates which are precipitated with lime is the high particle potentials in the critical pH range which adversely affects both particle size and settling rate.
Remove Arsenic by Precipitation as Barium Arsenate
The work of Chukhlansev (15) in 1956 showed that barium arsenate was perhaps the most insoluble of the simple metal arsenates. A process adopting the use of barium chloride to precipitate barium arsenate (incorporating radium) has been used in the Key Lake uranium process (24) by Key Lake Mining Corporation in association with Sherritt Gordon Mines Limited. Sherritt Gordon have patented a process for removing arsenic from aqueous solutions by using a soluble barium salt to precipitate barium arsenate (25). Arsenic (V) levels in solution can be reduced below 0. 1 mg/l in this process.
A process based on the precipitation of barium arsenate appears to be applicable to the treatment of arsenic from gold processing waste water. The high cost of reagents may be an important consideration.
Precipitation of Titanium (IV) – Arsenic (V) Compounds
The low solubility of titanium arsenates was indicated in a patent in 1932 (26). The Balkhash Mining-Metallurgical Combine (USSR) have since introduced a process for removing arsenic from copper electrolysis solutions (27) by precipitating TiOHAsO4 and a method for “regenerating” a “sorbent” (which is probably this compound) is described by Ugorets et al (28) .
Investigations in the author’s laboratory indicate a minimum solubility for a compound of Ti(IV)-As(V) of approximately 1 mg/l at a pH = 5.5.
Arsenic Precipitation of MgNH3AsO4.6H2O
In the Senken Process (29) after oxidative ammonia leaching of sulphide mineral, arsenic (V) is precipitated with MgSO4 as MgNH3AsO4.6H2O. Tozawa, Umetsu and Nishimura (30) found that this method of removing arsenic from solution was only effective in the range pH = 9.4 – 10.6 and the arsenic solubility in this region is about 50 mg/l.
Precipitation as Arsenic Sulphide
The Mining and Metallurgical Institute of Japan set up a study committee in 1975 to investigate the Full Utilization of Metallurgical Intermediates. The Chairman of that Committee, Professor Yoshio Kondo of the Department of Metallurgy at Kyoto University has reviewed (29) the processes used in Japan to treat arsenic in metallurgical processes.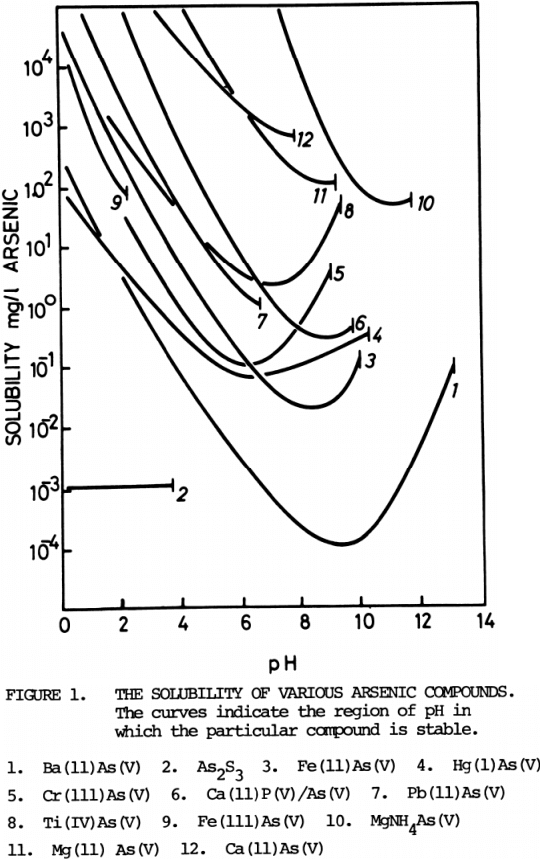
Arsenic is precipitated as As2S3 with either H2S or NaHS in a number of these processes. The processes are not related to gold processing but are applicable in that area.
The solubility of As2S3 can be calculated from thermodynamic data to be about 0.001 mg/l below pH = 4. The author (31) has produced “solubility” diagrams for the As-S-H2O which extend those produced by Tozawa et al (30) indicating the low solubility of As2S3 below pH = 4, but above that value the complex As3S6 -3 is the reason for As2S3 being soluble.
How to Remove Arsenic from Drinking Water
The presence of arsenic in the waste waters from the processing of gold bearing sulphides presents serious problems which are not easily overcome by simple precipitation techniques. Only three compounds of likely application to effluent treatment have direct arsenic solubility levels which are less than those which are prescribed in environmental standards; they are Ba3(AsO4)2, CaPO4/AsO4 and As2S3. Figure 1 compares the solubility of these compounds with others that have been used to reduce arsenic concentrations in aqueous solution.
The choice of a treatment method depends also on the oxidation state of arsenic in solution. Arsenic (111) can only be oxidised to arsenic (V) under fairly severe conditions such as those obtained using ozone or with high temperature – high pressure oxygen, so that the barium arsenate process, for example, would need an oxidation stage. Precipitation of As2S3 with H2S or other sulphides does not require the oxidation step but must be carried out below pH = 4 so could not be easily adapted to a final effluent polishing stage as could the barium process.
There is a great need for further investigations in relation to the removal of arsenic from process waste waters generally.
