Table of Contents
- Leaching Chemistry and Process Consideration
- Electrochemical
- Leaching
- Precious Metal Extraction
- Arsenic and Sulfur Separation and Recovery
- Epa-Tclp Test
- Analytical Methods
- Electrochemical
- Leaching of High-Grade Ore
- Rate of Arsenic Extraction and Activation Energy
- Precious Metal Extraction
- Leaching of Low-Grade Ore
- Arsenate Precipitation
- Sulfate Precipitation
- EPA-TCLP Test
- Flowsheet and Reagent Requirements
- Conclusions
Gold mining uncovers more and more hydrothermal deposits in which gold mineralization is associated with sulfides and other compounds of base metals, arsenic, antimony, or tellurium. This type of mineralization frequently makes gold recovery difficult by conventional techniques, such as amalgamation, gravity separation, or direct cyanidation. Environmental aspects of processing these ores associated with arsenic or other toxic metals need to be carefully considered. The U.S. Bureau of Mines investigated an alkaline oxidative leaching procedure for treating gold-bearing arsenopyrite (FeAsS) ore, as one of the continuing efforts to develop efficient and environmentally safe metallurgical processes to enhance domestic mineral productivity and efficiency.
Since a significant portion of gold occurs in a submicroscopic form and possibly as a lattice constituent of the mineral, liberation of gold requires breakdown of the crystal structure of the host minerals by oxidation. (2) Oxidation by roasting is no longer an acceptable method for arsenic-bearing minerals, such as arsenopyrite, in the United States because of environmental regulations regarding arsenic emissions (<0.5 mg/m³ air, standard temperature and pressure). (3) Hydrometallurgical processes are likely to be used to treat gold-bearing arsenical sulfide minerals. Earlier practice in recovering gold from arseno-pyrite has been reviewed by the Bureau, and a modified cyanidation-carbon adsorption method was developed to treat selected arsenopyrite concentrates (4). The procedure circumvents the problem of arsenic and sulfur emissions and is suitable for small gold producers.
Demopoulos and Papangelakis have reviewed the recent development of hydrometallurgical oxidation processes and their possible applications to treat refractory gold ores (5). These processes can be classified as pressure oxidation (6). biological oxidation (7). and chemical oxidation (8). Emerging pressure oxidation processes that can be used to treat gold-bearing arsenopyrite include a calcium chloride-oxygen leaching procedure (9) and the arseno process (10). The two procedures are unique in that mild temperatures ( < 115° C) and pressures ( < 100 psig O2) can be used to effect the oxidation of sulfide minerals. The arseno process involves the use of a catalyst.
A pressure oxidation procedure that has gained commercial status for treating gold-bearing arsenopyrite or pyrite employs sulfuric acid (H2SO4) and oxygen as reagents. High temperatures (170° to 190° C) and pressures (up to 400 psig O2) are required to achieve complete oxidation of the sulfide minerals. The chemistry of these reactions is complex. The dissolved arsenate precipitates as scorodite (FeAsO4 · 2H2O) and reports to a jarosite-bearing leach residue. However, controversy exists regarding scorodite’s environmental stability. The acid process necessitates that the residue be thoroughly washed and residual acid neutralized prior to cyanidation for the gold recovery. Ores containing high carbonates will result in high acid consumption. Finally, silver recovery from the procedure is usually poor because during oxidation silver has the propensity to combine with insoluble jarosites.
Alkaline pressure oxidation is not new; it was first proposed by Sill in the late 1950’s for treating arsenic sulfide ores and was used commercially for treating a cobalt-nickel arsenic ore. Alkaline pressure oxidation was also used to treat high-acid-consuming mercury gold ore. Alkaline pressure oxidation yields a residue composed primarily of iron oxide, which is environmentally safe and compatible with the cyanidation process. Solubilized arsenic and sulfur are recovered as relatively pure compounds and can be a source of raw material for insecticides in the agricultural or wood preservative industries and for drilling mud in the petrochemical industries. Recently, Taylor and Amoah-Forson investigated the hydrothermal oxidation of natural arsenopyrite in sodium hydroxide (NaOH) and the effects of several variables on the mineral. The effects of temperature and agitation at 40 to 200 psig O2 pressure and 0.01M to 1.0M NaOH solution on arsenopyrite dissolution were investigated. It was found that the reaction was diffusion controlled under the conditions investigated.
This report presents the results of bench-scale studies on the development of an alkaline oxidative procedure for pretreating gold-bearing arsenopyrite ore.
Leaching Chemistry and Process Consideration
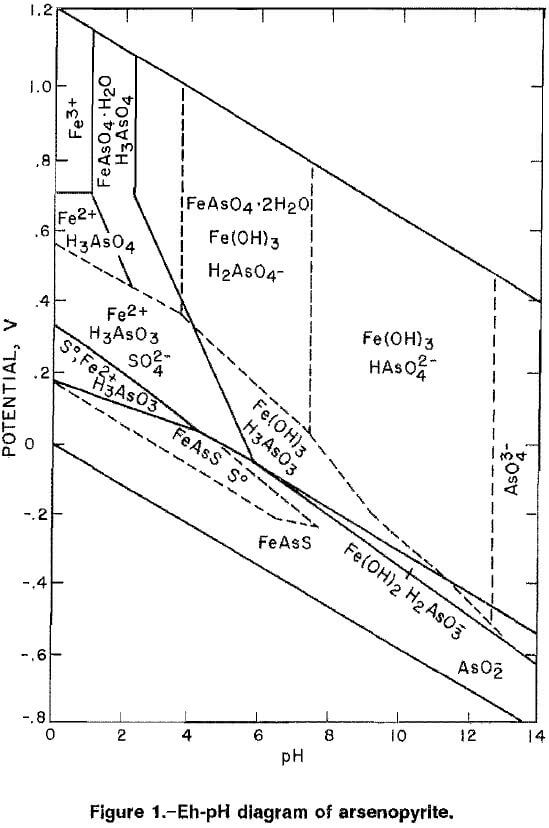
Oxidation occurring in alkaline media would be advantageous thermodynamically because lower potentials are required to oxidize arsenopyrite as indicated by the Eh-pH diagram for the Fe-As-S system in figure 1 (17). The alkaline oxidation of arsenopyrite can be represented by the following equation:
2FeAsS + 10OH + 7O2 = Fe2O3 + 2AsO4 3- + 2SO4 2- + 5H2O……………………..(1)
The arsenate and sulfate can be carried away from the reaction surface by agitation, but the iron oxide produced could form a layer over the unreacted arsenopyrite. Thus, the alkaline leaching system to be studied for arsenopyrite oxidation should be one that minimizes the harmful effect of iron oxide.
Cyclic voltammetry and chronocoulometry can be used to determine the relative reaction rates of electronic conductors, such as arsenopyrite, as a function of potential in various alkaline media. Cyclic voltammetry has been used previously to study the dissolution of arsenopyrite in basic solutions. Chronocoulometry measures any change in current density or reaction rate over time at a set potential. The reaction decay rate demonstrates the effect of adhering reaction products.
Once an alkaline medium is selected, based on electrochemical studies, the effects of base concentration, temperature, oxygen pressure, percent solids, and reaction time on extraction of arsenic from arsenopyrite need to be investigated. Since the ultimate purpose of the pretreatment is to enable extraction of precious metal values, the relationship between the extent of arsenic extraction from gold ores and subsequent precious metal extraction by cyanidation needs to be investigated.
Dissolved arsenate and sulfate in the leaching solution must be removed to partially regenerate the NaOH and facilitate solution recycle to the oxidative leach. All waste products of the process would have to pass the Environmental Protection Agency’s guidelines on solubilities of toxic substances as outlined in the Toxicity Characterization Leaching Procedure (EPA-TCLP) test procedures (20). A process flowsheet integrating the alkaline oxidative leaching, arsenic precipitation, sulfate precipitation, and extraction of precious metals would be developed based on experimental results.
Experimental Procedures
Electrochemical
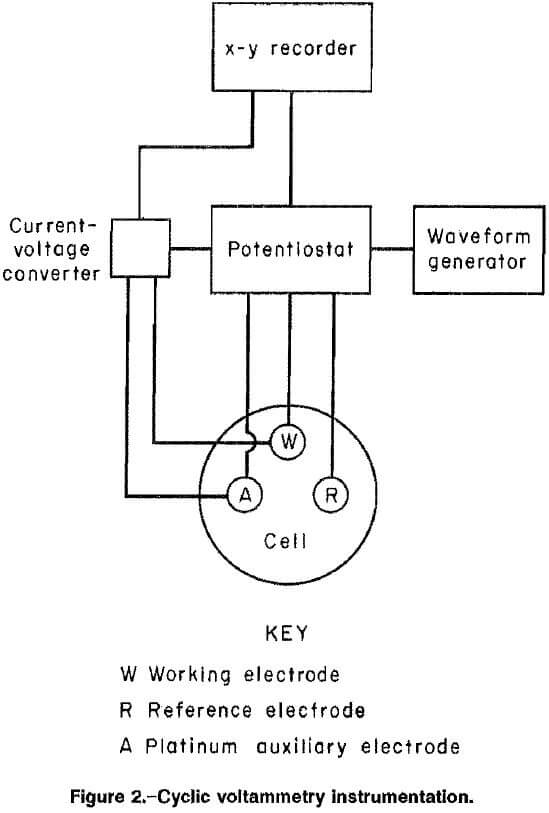
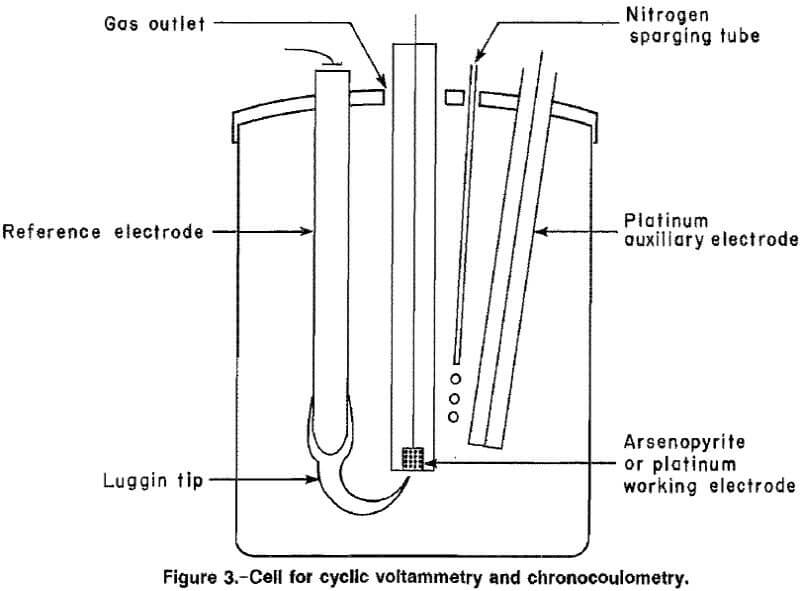
Instrumentation used for the cyclic voltammetry tests is shown in figure 2. The apparatus included a potentiostat, a programmable waveform generator, a current-to-voltage converter, a standard three-electrode cell, and an x-y recorder. An internal resistance compensator was used to reduce effects of parasitic resistances in the various basic solutions. Chronocoulometric tests were conducted with the same equipment and a computing coulometer. A standard three-electrode cell, with a working volume of 100 mL, (fig. 3), was used for all the tests. The cell was a wide-mouth glass jar with four inlets through the lid for a working electrode, a saturated calomel reference electrode, a platinum auxiliary electrode, and nitrogen purge.
Two types of working electrodes were used: a commercial disk-type platinum electrode with a 20.1-mm² area and a pure, natural arsenopyrite electrode. The arsenopyrite specimen was attached to a drilled and tapped copper plug using a nickel-base conductive cement. This assembly was then encased in epoxy, leaving the threads on the copper plug exposed. The mineral face was polished to expose the working surface, while the threaded end of the copper plug provided an electrical contact through a rotating shaft.
The working surface of the arsenopyrite electrode was polished with 1-µm, diamond paste before each test to minimize surface condition effects, The electrode area was measured using a digital analysis system (21) before each test to account for area differences arising from polishing of the irregular surface.
Oxygen was eliminated from the solution by purging with high-purity nitrogen (99.995 pct) prior to the tests and maintaining a nitrogen blanket above the solution during the tests. All electrochemical tests were conducted at ambient temperature (-23° C) using solutions prepared with reagent-grade chemicals and triple-distilled deionized water. All cyclic voltammetry tests were conducted with 20 mV/s scan rate and initiated at the open-circuit potential, inducing oxidizing potentials up to 0.842 V, then reducing potentials to -0.558 V, and finally terminating the cycle at the rest potential. All potentials in the text have been converted from the saturated calomel scale and are referenced to the standard hydrogen electrode (SHE). Background currents from the solution were determined using the platinum working electrode and were subtracted from the arsenopyrite tests. Chronocoulometric tests were conducted at a set potential as determined from the cyclic voltammetry tests, and the current density was measured versus time.
Leaching
Leaching was conducted in a standard 2-L AISI Type 316 stainless steel autoclave equipped with a 1.5-kW heater and 1/15-hp stirring motor. The head incorporated a magnetic-drive stirring assembly, a 600-psig pressure gauge, a thermowell, a solution-sampling tube, and a gas inlet and outlet. The stirrer consisted of a 2-in, six-blade, turbine-type impeller positioned at the end of the stirrer shaft and near the bottom of the vessel. Stirring speed was 600 rpm. The solution-sampling tube was equipped with a dual-disk stainless steel filter capable of retaining materials larger than 10 µm.
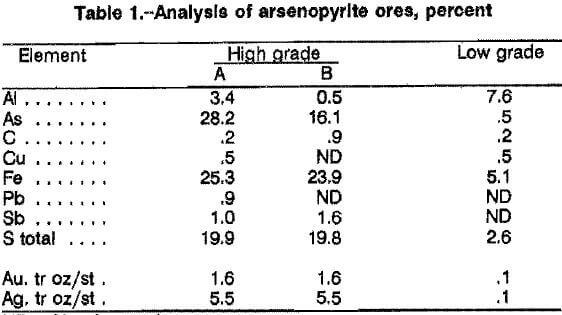
Analyses of the two high- and one low-grade arseno-pyritic gold ores, minus 35 plus 400 mesh, are listed in table 1. The principal difference between the high-grade ore A and ore B was the arsenopyrite and pyrite contents. Ore A contained approximately 61 pct arsenopyrite and 9 pct pyrite, and ore B contained 35 pct arsenopyrite and 24 pct pyrite. The low-grade ore contained only 1 pct arsenopyrite and 5 pct pyrite. Scanning electron microscopy analysis and X-ray diffraction patterns of the ores indicated that virtually all of the arsenic was present as arsenopyrite. The high-grade gold ore A was used in all of the experimental work performed to determine best leaching conditions. Ore B was used for all flowsheet mass balance and recycle experiments. All chemicals used were reagent grade. Solutions were made with deionized water.
Freshly prepared NaOH solution at 32° to 38° C (resulting from the exothermic dissolution of the NaOH in water) and ore were added to the reactor. The reactor was sealed, pressurized with oxygen, and heated to operating temperature in 30 to 45 min, with intermittent stirring during heating to minimize reaction and achieve uniform temperature. Once the system reached operating temperature, the slurry was stirred continuously. At the end of the experiment, the slurry was vacuum filtered. The residue and the leach solution were analyzed for arsenic, sodium, iron, total sulfur, sulfate, and hydroxide.
The leaching parameters and ranges investigated were- NaOH concentration, 0.5M to 2.0M; temperature, 80° to 140° C; oxygen pressure, 40 to 200 psig; pulp density, 10 to 20 pct solids; and reaction time, 2 to 7 h. Solution volume was 500 mL. Leaching rates were determined at 80°, 100°, and 140° C by arsenic analysis of 2-mL samples taken at 1-h intervals after the system reached operating temperature.
Precious Metal Extraction
The alkaline leach residue was cyanide leached at 10 pct solids using 0.3 g NaCN (sodium cyanide) per 20 g alkaline leach residue (15.0 kg NaCN per metric ton alkaline leach residue). The slurry was placed in a 2-L bottle and agitated by rolling at 100 rpm for 24 h. The slurry was vacuum filtered, and the solution and residue were analyzed for gold and silver. Based on these analyses, the percent extractions of gold and silver were calculated,
Arsenic and Sulfur Separation and Recovery
Dissolved arsenic in the oxidative leach solution was precipitated as calcium arsenate [Ca3(As04)2] at 20° to 30° C by adding lime (CaO). Volumes of 500 to 2,000 mL of leach solution were treated with 50 g/L CaO in a 2-L beaker and stirred at 300 rpm with a 2-in-long magnetic bar for 30 min. The slurry was filtered using Whatman No. 5 filter paper, and the cake was dried for 4 to 6 h at 90° C. The filtrate was used subsequently in sulfate removal experiments.
Two sulfate precipitation methods were investigated for removing sulfur from the filtrate. First, sulfate as calcium sulfate (CaSO4) was precipitated by adjusting the solution’s pH to 6, using either sulfuric or acetic acid and adding lime. Second, sulfate as barium sulfate (BaSO4) was formed at 20° and 45° C at pH 12 by adding barium hydroxide monohydrate [Ba(OH)2·H2O]. A stoichiometric amount of Ba(OH)2·H2O was added to the solution and stirred for 30 min. The precipitate was filtered with Whatman No. 5 paper.
Epa-Tclp Test
The cyanide leach residue and the Ca3(AsO4)2, BaSO4, and CaSO4 precipitates were tested to determine if standards for nonhazardous waste would be met using EPA guidelines. One hundred grams of each solid was added to 2 L of solution containing 5.7 mL acetic acid per 994.3 mL deionized water. The slurry was rolled for 18 h and filtered using Whatman No. 5 paper, and the filtrate was analyzed for arsenic, silver, lead, and barium.
Analytical Methods
Metals in the solids and solutions were analyzed by inductively coupled plasma spectroscopy. Hydroxyl ion concentration of the solutions was determined by equilibrium titration with standardized acid. Sulfate and total sulfur were determined by wet chemical analysis, and precious metals by fire assay and atomic absorption spectroscopy. X-ray fluorescence, X-ray diffraction, and infrared spectroscopy were used to examine the ores, leached residues, and electrochemical test residues where appropriate.
Results and Discussion
Electrochemical
The Eh-pH diagram, (fig. 1), indicates that arsenopyrite will oxidize at lower potentials as the pH is increased. The results of cyclic voltammetry for arsenopyrite in different basic solutions, as shown in table 2, agree with this prediction. The initial potential, the potential at which the current for oxidation of arsenopyrite exceeded the background current by more than 0.001 mA, was 0.10 V in 0.5M NH4OH (ammonium hydroxide) plus 0.5M (NH4)2CO3 (ammonium carbonate) at pH 9.6, 0 V for 1.0M NH4OH at pH 12, and -0.18 V for 1.0M NaOH and 1.0M NaOH-0.1M NH4OH at approximately pH 13. The open-circuit potential also followed this pattern, decreasing from 0.044 V to -0.183 V with an increase in pH from 9.6 to 13.2. All basic solutions tested were capable of supporting the oxidation of arsenopyrite at low potentials. Changes in the current densities with each cyclic scan are indicative of kinetics for the oxidation of arsenopyrite in different basic solutions. The data in table 3 show the changes of current densities in the first and fifth cycle at potentials of 0.242 V and 0.642 V. At 0.242 V, the current density was highest for the NaOH, followed by NaOH-NH4OH, NH4OH-(NH4)2CO3, and NH4OH, The current density increased in all solutions when the potential was increased to 0.642 V, with the highest current density recorded in the NaOH solution and the lowest in the NH4OH solution.
A decrease in the current density with subsequent cycles was noted in all of the solutions. At 0.242 V, the largest decrease in current density occurred with NH4OH- (NH4)2CO3, followed by NH4OH, NaOH-NH4OH, and NaOH. The decrease with the last solution was less than 1 pct. The decay in the current densities in repeated scans was attributed to the formation of a brown film on the surface of the electrode, which can prevent diffusion of chemicals and hinder arsenopyrite oxidation. X-ray diffraction and infrared spectroscopy analyses showed the film to be an amorphous hydrous ferric hydroxide. Increasing the potential can be a method for preventing the current from decaying and, thus, overcoming the reaction barrier caused by the iron film, as illustrated with the NH4OH-(NH4)2CO3 and the NaOH-NH4OH solutions. The current density in the NH4OH-(NH4)2CO3 solution decreased to undetectable levels after the fifth scan at 0.242 V but decreased by only 14 pct at 0.642 V. Similarly, the current density in the NaOH-NH4OH solution decreased by only 8 pct at 0.642 V compared with a 24-pct decrease at 0.242 V after the fifth cycle.
Chronocoulometric tests were conducted at 0.242 V to determine the effect of the iron film on the oxidation of arsenopyrite over an 8h period in the NH4OH-(NH4)2CO3, NaOH, and NaOH-NH4OH solutions. The film that formed in the carbonate solution was the most detrimental, with oxidation stopping completely after the first 5 min of reaction. Current densities in the NaOH and NaOH-NH4OH systems decreased by 22 pct and 45 pct, respectively, during the first hour but reached a steady state thereafter. These results suggest that the films formed on the partially oxidized arsenopyrite might be impervious, because a passive film would completely stop the reaction. Instead, the current density was only hindered, indicating the reaction was limited by the diffusion of reactants or products through a porous film. The NaOH solution supported the highest degree of oxidation with 0.659 C/mm² after 8 h, followed by the NaOH-NH4OH solution with 0.162 C/mm², and the NH4OH-(NH4)2CO3 solution with 2.67 x 10 -5 C/mm².
Data from the electrochemical studies indicate that NaOH is the best solution for oxidative pretreatment of gold-bearing arsenopyrite ores because it promoted the oxidation of arsenopyrite at the lowest potentials with a rate proportional to the applied voltages. The hydrated iron oxide film on the partially oxidized arsenopyrite in the NaOH solution appeared to be porous, allowing a steady-state reaction with the highest coulombic throughput per unit area.
Leaching of High-Grade Ore
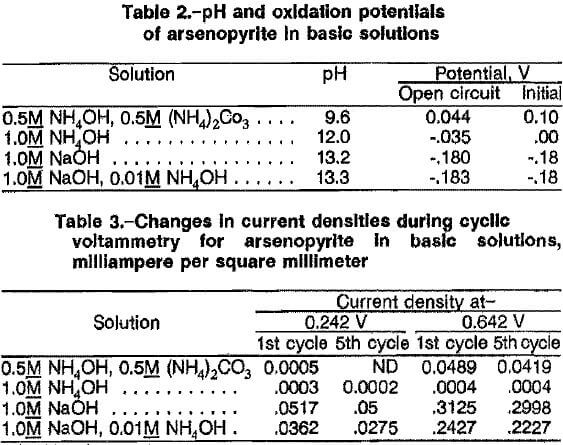
The effects of NaOH concentration, oxygen pressure, temperature, reaction time, and percent solids on extraction of arsenic from ore A are given in table 4. The ore contains 61 pct arsenopyrite, and the stoichiometric NaOH for complete extraction of arsenic is 1.88 mol of NaOH per 100 g ore according to equation 1. Table 4 indicates that more than stoichiometric NaOH was required to obtain high extraction of arsenic. Tests 1, 2, and 3, used 27, 53, and 106 pct of stoichiometric NaOH, respectively. Extractions of 29, 54, and 81 pct arsenic were achieved at 140 psig 02, 140° C, and 2 h when these concentrations of NaOH were used. The excess NaOH required was attributed to consumption of the reagent by pyrite and other metal sulfides in the ore.
Data for tests 4 and 5, which used 106 pct of stoichiometric NaOH (2.0M), 140 psig O2, and 7 h, indicate that the extraction of arsenic increased from 85 pct at 80° C to 95 pct at 100° C. However, the extraction of arsenic decreased to 91 pct at 140° C (test 6). The decrease in arsenic extraction at 140° C can be explained by the precipitation of arsenate as sodium arsenate. The higher temperature increases the rate of arsenate extraction, resulting in saturation of the leach solution with arsenate.
Data for tests 7 and 8, which used 106 pct of stoichiometric NaOH (2.0M) and a leaching temperature of 100° C, show that the arsenic extraction of 91 pct at 40 psig O2 was the same as that at 140 psig O2. The extraction of arsenic in test 9 decreased to 86 pct at 200 psig O2. The reason for the decrease in arsenic extraction at > 140 psig O2 pressure was not apparent. Lower oxygen pressure favored higher extraction of arsenic.
The effect of percent solids on arsenic extraction using 106 pct of stoichiometric NaOH (2.0M)> 140 psig O2, 100° C, and 4 h is illustrated by the results from tests 8, 10, and 11. The extraction of arsenic was 91 pct at 10 pct solids (test 8), 87 pct at 15 pct solids (test 10), and 79 pct at 20 pct solids (test 11). The results indicate that leaching conducted with more than 15 pct solids decreased the extraction of arsenic substantially.
Increasing the leaching time generally resulted in higher arsenic extractions, as indicated by comparing the results from tests 3 and 6, and 5 and 8. The ideal leaching time was defined as that needed to extract enough arsenic such that a high extraction of gold would result from the cyanidation of the oxidative leach residue. The overall results indicate that the best leaching conditions for extraction of more than 90 pct arsenic were 2M NaOH, 100° C, 40 psig, 100 g/L ore, and 4 to 7 h.
The leach residue was reddish brown in color and easy to filter. Analysis of the residue by X-ray diffraction showed an amorphous solid, but infrared spectroscopy identified iron oxide with varying degrees of hydration depending on the oxygen pressure used in the leach. As the oxygen pressure increased, the residue approached a hematite structure.
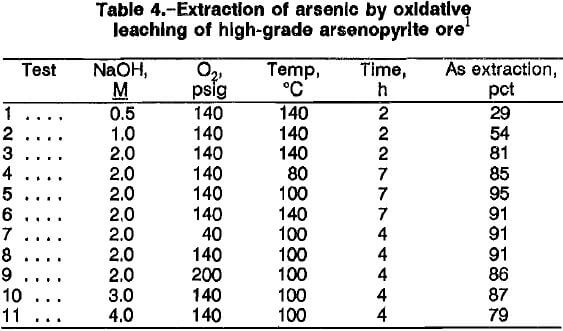
Rate of Arsenic Extraction and Activation Energy
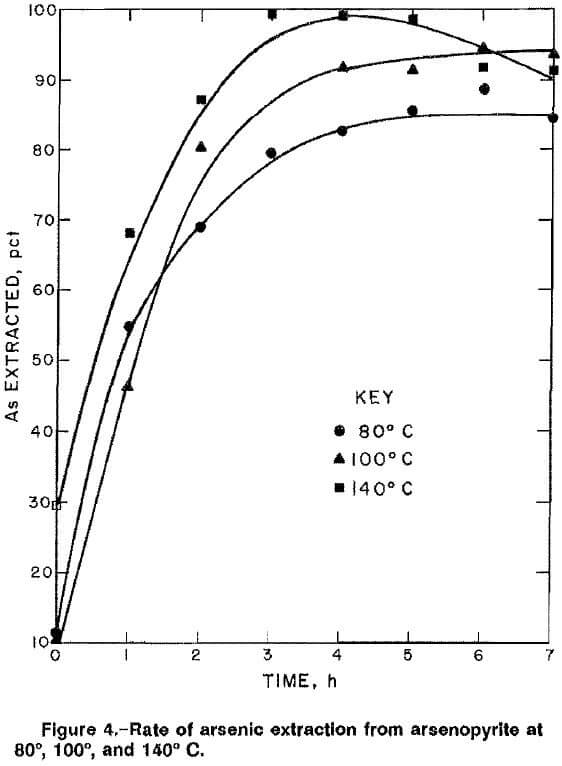
The rates of arsenic extraction for high-grade ore A in a 2M NaOH solution at 140 psig O2 were determined at 80°, 100°, and 140° C (fig. 4). The rates for all three temperatures were rapid during the first 4 h of reaction. During the last 3 h, the extraction rate decreased at 140° C, and marginal rate increases were noted at 80° and 100° C. The decrease at 140° C might be attributed to precipitation of sodium arsenate. An Arrhenius plot (fig. 5) of the reaction rate constants obtained for the first 4 h of reaction gives an activation energy of 20.3 kJ (4.8 kcal) per mole. The value suggests that diffusion of reactants may be the rate-controlling factor, which in this case is the diffusion of NaOH and/or oxygen through the hydrous iron oxide layer on the partially reacted arsenopyrite. The activation energy value of 20.3 kJ/mol was lower than that obtained by Taylor and Amoah-Forson, who reported an activation energy of 23.8 kJ (5.7 kcal) per mole for the dissolution of natural arsenopyrite in NaOH solution.
Precious Metal Extraction
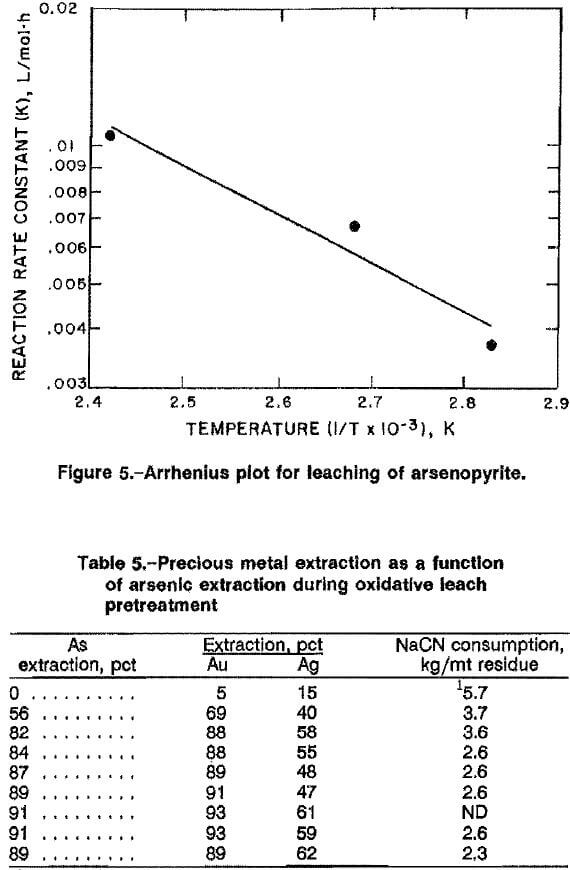
Cyanidation of high-grade ore A resulted in 5 pct Au and 15 pct Ag extraction, using 15 kg NaCN per metric ton ore, Cyanidation of the oxidative leach residue with 15 kg NaCN per metric ton residue improved precious metal extraction, as shown in table 5. Gold recovery was proportional to arsenic extraction during the oxidative leach, with >90 pct Au extraction for arsenic extraction ≥ 89 pct. Silver extraction did not follow this trend and ranged from 47 to 62 pct. Cyanide consumption was 2.6 kg NaCN per metric ton of leach residue and 5.7 kg NaCN per metric ton for the untreated ore.
Leaching of Low-Grade Ore
Leaching 50 g of the low-grade ore in 500 mL of 2M NaOH solution at 140 psig O2, 100° C, and 4 h resulted in 90 pct extraction of arsenic. The leach residue, a gelatinous material formed probably because of the clays present in the ore, was difficult to filter. Subsequent cyanidation of the leach residue resulted in 70 pct Au and 31 pct Ag extraction compared with <20 pct Au extraction using direct cyanidation.
A flotation concentrate was made using sodium isopropyl xanthate as collector and copper sulfate (CuSO4) as activator. It contained 5.6 pct arsenopyrite, 32.2 pct pyrite, 0.89 tr oz/st Au, and 0.55 tr oz/st Ag. Eighty-three percent of the gold and 53 pct of the silver were floated. Leaching of the flotation concentrate at 100° C, 2M NaOH, 10 pct solids, 140 psig O2, and 4 h resulted in 96 pct extraction of arsenic, and more significantly, the residue was easy to filter. Cyanidation of this residue resulted in 92 pct Au and 63 pct Ag extraction. The overall extraction was 76 pct for gold and 33 pct for silver. The cyanide consumption for the flotation concentrate was 3 kg NaCN per metric ton of leach residue.
Arsenate Precipitation
The leach solution contained 13 to 27 g/L As, 14 to 17 g/L S, 8 g/L OH, 36 to 48 g/L Na, <1 ppm Fe, 29 to 60 ppm Sb, <8 ppm Ca, and 160 to 280 ppm Si, concentration variabilities caused by the changes in composition of the high-grade ores. Arsenic was removed by precipitation in order to produce a solution that could be recycled to the oxidative leach. A buildup of arsenate in the leach solution would result in <90 pct. As extraction because the solubility limit for arsenate would be exceeded. Thus, arsenate is precipitated from solution, using twice the lime predicted by equation 2:
4CaO + 2Na3AsO4 + 4H2O = Ca(OH)2+ Ca3(AsO4)2 + 6NaOH………………………….(2)
Ninety-eight percent of the arsenic was precipitated from the solution. The precipitate contained 20 to 36 pct Ca, 11 to 17 pct As, 0 to 1.5 pct S, 5 to 7 pct Na, 200 to 400 ppm Fe, and 400 to 600 ppm Sb.
Sulfate Precipitation
Since <5 pct sulfate is precipitated at pH 12 during arsenate removal, a separate precipitation step is necessary to remove the remainder of the sulfate before the solution is recycled. The precipitation of sulfate with lime was tried using a combined filtrate collected from various arsenic precipitation tests. The solution, pH 12 to 13, contained 200 to 500 ppm As and 10 to 12 g/L S. The pH was adjusted to pH 6 with either H2SO4 or glacial acetic acid (HOAC). The results of the tests are given in table 6. The HOAC-adjusted tests resulted in a higher sulfate precipitation than the H2SO4-adjusted tests. The precipitates were easy to filter and contained calcium hydroxide [Ca(OH)2] as a major impurity. The residual arsenate in the leach solution is scavenged during the sulfate precipitation, resulting in less than 1 pct As remaining in solution. The hydroxide concentration in the final filtrate was less than 1 g/L; therefore, 2 mol NaOH would have to be added to the solution before it could be recycled. The lack of hydroxide regeneration is the major disadvantage of using lime for sulfate precipitation.
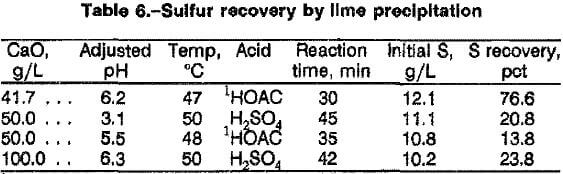
In an effort to decrease the total sulfur Concentration to less than 1.0 g/L and replenish part of the hydroxide consumed during the oxidative leach, Ba(OH)2·H2O was added to the filtrate after Ca3(AsO4)2 precipitation to precipitate BaSO4. This solution contained, in parts per million, 200 to 2,200 As, <8 Sb, <1 Fe, <3 Pb, <30 Si, and 350 Ca and, in grams per liter, 23 to 45 Na, 14 to 23 S, and 8 to 11 OH. Approximately 90 to 100 g/L of Ba(OH)2 was required to precipitate more than 96 pct of the sulfur as BaSO4 from the solution. After the precipitation, the filtrate contained 0.6 to 1.0 g/L total sulfur. The precipitate formed at 45° C required less time to filter than the 20° C precipitate. If the 20° C precipitate was allowed to settle before filtering, a major portion of the solution could be decanted, and the filtration time decreased significantly. The BaSO4 precipitate contained barium carbonate (BaCO3) as the major contaminant. A typical assay of the precipitate was, in parts per million, <50 Fe and <320 Sb and, in percent, 0.5 Ca, 1.6 Na, 12 S, and <0.02 to 4.4 As. Washing the precipitate once with water and twice with a 0,6-pct acetic acid solution, successively, reduced the BaCO3 and entrapped arsenic contents. The precipitate after the second acetic acid wash was 99.5 pct BaSO4.
EPA-TCLP Test
The cyanide leach residue and Ca3(AsO4)2, BaSO4, and CaSO4 precipitates were subjected to the TCLP test to determine if the solids met the EPA nonhazardous waste standards for arsenic, silver, and lead. The limit for arsenic, silver, and lead is 5 ppm. The cyanide leach residue effluent analysis showed 1.8 ppm As, <1 ppm Ag, and <3 ppm Pb. The effluent for Ca3(AsO4)2 and CaSO4 precipitates analyzed < 1 ppm As. There was no silver or lead in the precipitate. Thus, these residues can be classified as nonhazardous under EPA regulations. However, the long-term stability of the Ca3(AsO4)2 is not known. The BaSO4 precipitate did not meet either the TCLP effluent barium limit of 100 ppm or the arsenic limit until it was washed with water followed by two washes with 0.6-pct acetic acid solution.
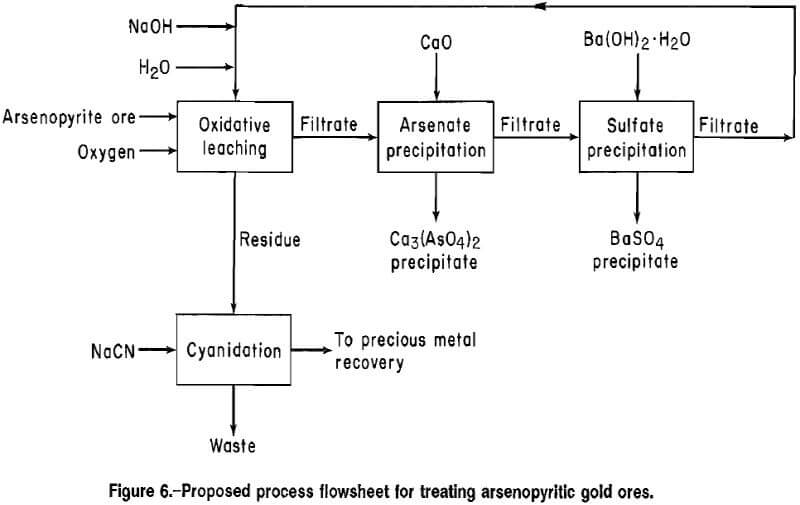
Flowsheet and Reagent Requirements
A flowsheet for treating high-grade arsenopyritic gold ores, based on experimental results, is shown in figure 6. The arsenopyrite and pyrite are oxidized by an alkaline oxidative leach, and the precious metals are extracted by cyanidation of the leach residue. The alkaline leach solution is purified by precipitating arsenate and sulfate in successive steps and is recycled to the oxidative leach.
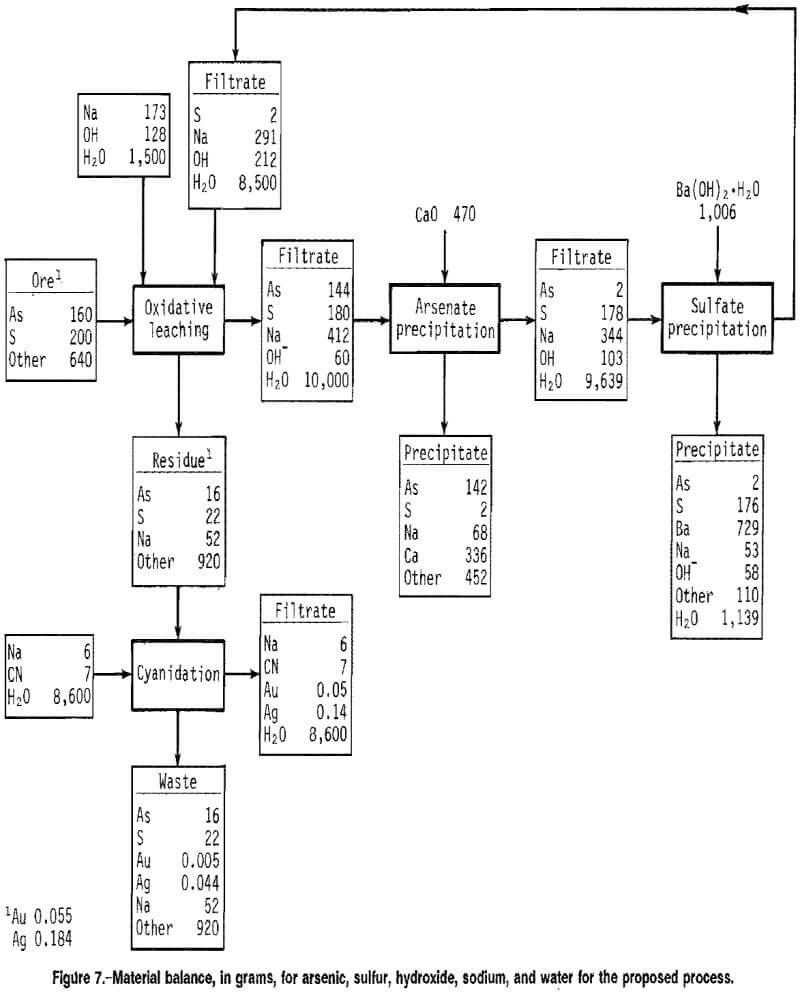
Material balances, in grams, for arsenic, sulfur, sodium, hydroxide, and water are shown in figure 7 for each unit operation, based on cyclic test data for 2M NaOH, 10 pct solids, 100° C, 40 psig O2, and 4 h for the alkaline leach of ore B. The data shown represent values that were in the range of values found during repeated testing of the process. Losses due to sampling and transfer of materials were assumed to be zero in order to balance the flowsheet. The material balance shows that 90 pct of the arsenic and sulfur were extracted. Cyanidation of the leach residue resulted in 91 pct Au and 76 pct Ag extraction. Addition of a 100-pct excess of lime precipitated over 96 pct of the arsenate from solution. The excess calcium precipitated as Ca(OH)2. More than 98 pct of the sulfate was precipitated with the stoichiometric addition of Ba(OH)2 · H2O. The filtrate recycled to the leach contained 1.5M NaOH.
The principal advantage of the alkaline oxidative leaching for arsenopyritic gold ores is the use of mild operating temperatures and pressures corresponding to lower energy requirements than for acidic oxidative leaching. Furthermore, the leach residue can be cyanided directly without pH adjustment. The cyanide leach residue contains hydrated iron oxides and silica, which are nonhazardous. The relatively pure arsenate waste may be a marketable item. The sulfate precipitate can be washed with water followed by a 0.6-pct acetic acid solution to extract the BaCO3 and residual arsenic before disposal or marketing.
The disadvantage of the process is the use of relatively expensive reagents such as NaOH for the oxidative leach and Ba(OH)2-H2O for sulfate control. Hydroxide consumption is dependent on the arsenopyrite and pyrite content of the ore. From figure 7, hydroxide consumption during the oxidative leach is calculated to be 260 kg/mt of this particular ore. Methods for decreasing the hydroxide requirement and for controlling sulfur in concentrated basic solution should be the thrust of future research.
Conclusions
The best solution composition for arsenopyrite oxidation was determined with cyclic voltammetry techniques, NaOH is the best basic leaching solution because of the low oxidation potential requirement of -0.20 V versus SHE and relatively high oxidation rates, Chronocoulometric studies indicated that the amorphous hydrous iron oxide formed on the partially oxidized mineral in NaOH solution does not passivate the reaction.
The alkaline oxidative leach pretreatment for gold-bearing arsenopyrite ores uses mild conditions, and the leach residue requires no washing or pH adjustment before cyanidation. By using 2M NaOH, 100° C, 40 psig O2, 10 pct solids, and 4 h, more than 90 pct of the arsenic was extracted from the high-grade ore. The gold and silver extractions were 90 and 76 pct, respectively, by cyanidation of the leach residue. When the leaching procedure was applied to a concentrate prepared from an ore containing 0.1 tr oz/st Au and 0.1 tr oz/st Ag, the gold extraction was 76 pct and the silver extraction was 33 pct. The soluble arsenate and sulfate were successfully removed from the pregnant solution as separate products. All the residues passed the EPA TCLP tests for arsenic, except BaSO4. As a result of these studies, an alkaline oxidative leaching procedure for pretreating arsenopyrite ores for extraction of precious metals was developed.

