Table of Contents
Current practice for the production of wet-process phosphoric acid (H3PO4) involves leaching beneficiated phosphate rock with aqueous H2SO4. Typical processing involves mining the ore, also called matrix, using a dragline, then forming a water-based slurry to facilitate pumping the ore to a beneficiation plant. Phosphate values contained in the minus 150-mesh fraction of the ore slurry cannot be economically recovered. Normal practice is to discard this fine particle fraction, called phosphatic clay waste, by pumping it into waste ponds. This clay waste contains approximately one-third of the total phosphate originally present in the matrix. The rate of flow of this fine-particle waste slurry into waste ponds ranges between 15,000 and 80,000 gal/min and its solids content is only 2 to 6 pct. Since the waste is a colloid-like suspension, the rate of settling is slow and after years the material still contains more than 70-pct water. The individual ponds that accommodate these wastes average 400 to 600 acres with dam heights ranging from 20 to 60 ft. Thus, millions of tons of unrecovered phosphate are discarded, tying up large quantities of land and water. Unfortunately, the established methods for reclaiming these phosphatic waste ponds require decades for adequate consolidation to allow any attempts at phosphate recovery. However, a new “ditching” technique, developed by Agrico Mining Co., has reduced reclamation time to only 3 to 5 years. As a result, large quantities of dried clay waste have become potentially available. Recovering phosphate from these fine-particle wastes would substantially increase the domestic reserve.
One approach that has been investigated for increasing the recovery of phosphate is direct leaching of phosphate from matrix.
Although phosphate extractions as high as 90 pct have been reported using H2SO4 as the leaching agent, this direct acidulation of matrix also extracts unacceptably high levels of iron and aluminum. These impurities render the wet-process acid produced unsuitable as a precursor for making fertilizer. However, much work has been done on purifying wet-process acid by extracting the H3PO4 contained therein using organic solvents. In fact, some researchers have reported leaching phosphate values in the presence of an organic solvent; the organic rejects hydrophilic impurities, thereby enhancing the purity of the crude acid product. This kind of technique, essentially a combination of direct acidulation and solvent extraction, might have the potential to reduce phosphate losses from current matrix processing, and it might also be used to recover phosphate values from the large tonnages of material in existing waste ponds.
A leaching technique being investigated by the Bureau involves direct acidulation of methanol-based slurries of waste clays using H2SO4 as the lixiviant. The majority of waste clay samples tested produced phosphate extractions in the 60- to 70-pct range. Complete extraction of phosphate values was not achieved even when excess H2SO4 was used and long reaction times were employed. Although a preliminary internal economic evaluation of the acid-alcohol technique was favorable, an improvement in extraction levels was desired. Since apatite is the dominant phosphate-containing mineral in the waste materials, a study was conducted using research-grade apatite, devoid of any ancillary clays and minerals. Using the crystalline apatite in the acid-alcohol leaching tests provided evidence as to why the extractions are incomplete.
Identification of a Coating on Acid-Alcohol Treated Apatite Crystals
Conventional processing of phosphate rock with H2SO4 may induce a gypsum coating on the surface of the phosphate particles, especially if the initial particle size is too coarse. This results in decreased phosphate recovery since the coated particles are rendered inert to further reaction with acid. In the leaching of phosphatic clay wastes using H2SO4-methanol it was observed that, after treatment, unreacted apatite was present in the leached tailings and unreacted H2SO4 remained in solution. Thus, a coating phenomenon similar to that observed in conventional processing was suspected. Unfortunately, because of the large amounts of gangue (e.g., clays, sand) present in phosphatic clay wastes, a detailed surface analysis of the leached tailings would be difficult. Therefore, to investigate potential causes for the incomplete extractions, leaching studies were done using research-grade fluorapatite. Unlike phosphatic wastes, the pure apatite contained no accessory clays or other minerals. Thus, this provided an ideal system in which to study H2SO4-methanol leaching of phosphate-containing mineral without the presence of interfering side reactions between acid and siliceous gangue.
Preparation and Description of Apatite
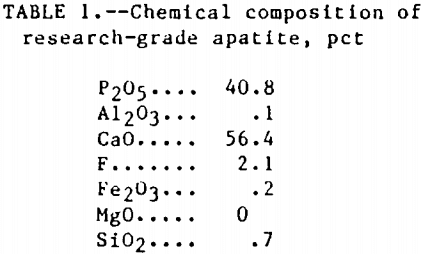
A sample of research-grade apatite was obtained from Ward’s National Science Establishment, Inc. This sample was obtained in the form of green crystal prisms, ranging in size from ½ to ¾ in. Ward’s obtained the apatite from a deposit in Wilberforce, Ontario. The apatite was ground in a rotary mill and the minus 28- plus 100-mesh fraction was used in this study. A chemical composition analysis was conducted on the apatite sample and is presented in table 1.
Acid-Apatite Ratios for Leaching Apatite
The pure apatite crystals used in this study, as indicated by XRD, are primarily of the fluorapatite form. The chemical reaction that takes place when fluorapatite is treated with H2SO4-methanol is as follows :
Ca5(PO4)3F + 5H2SO4 + 5/2H2O → 3H3PO4 + 5(CaSO4·½H2O) + HF
Therefore, in this study the acid-to-apatite ratio (acid-Ap) was held stoichiometrically at 5:1. Because of the presence of the organic solvent, the precipitate formed during acid-alcohol leaching is calcium sulfate hemihydrate, CaSO4·½H2O, not gypsum.
Equipment and Procedures
Leaching experiments were conducted in a 3-neck, 500-mL, round-bottom flask, equipped with an overhead stirrer and reflux condenser. A Metrohm 665 Dosimat was used to add H2SO4 to the flask. Experiments were conducted using 25 g of apatite and 50 mL methanol. The flask contents were mixed vigorously while adding the desired quantity of technical grade H2SO4 (93 pct) over a 30-min period. After the acid addition was completed the reaction mixtures were stirred for another 30 min. Slurries were vacuum-filtered, using a laboratory aspirator, through Whatman No. 3 filter paper to separate leaching liquors from tailings. Filter cakes were washed twice with 50-mL aliquots of methanol and then dried in an oven at 110° C. Elemental analyses were obtained on leaching liquors, washing solutions, and filter cakes. Petrographic analyses of apatite samples, before and after leaching, were done using a Leitz Metzlar optical microscope with camera attachment. These samples were also analyzed in the infrared range using a Nicolet 740 FT-IR spectrometer. The surface morphology of samples was studied using a Hitachi S-2300 SEM.
Results
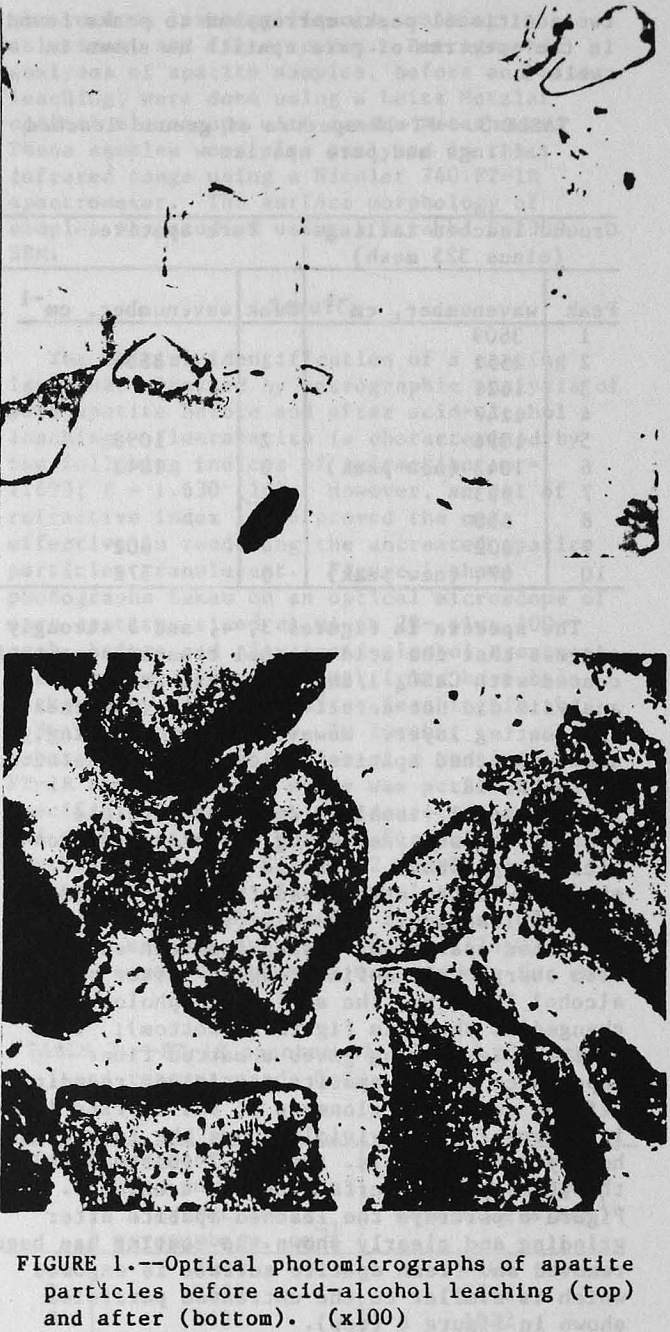
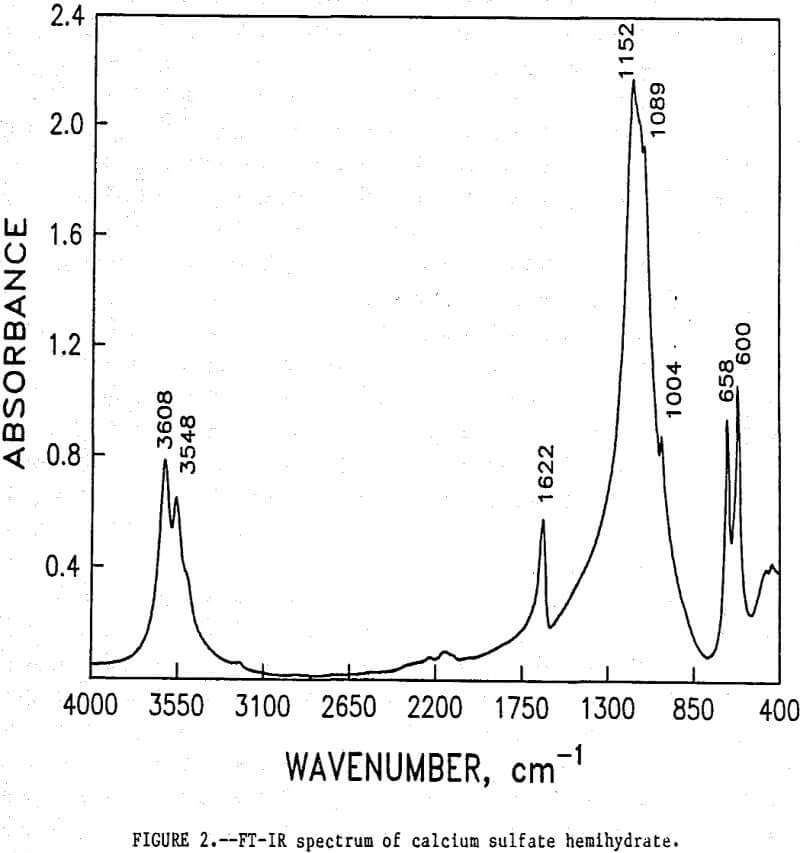
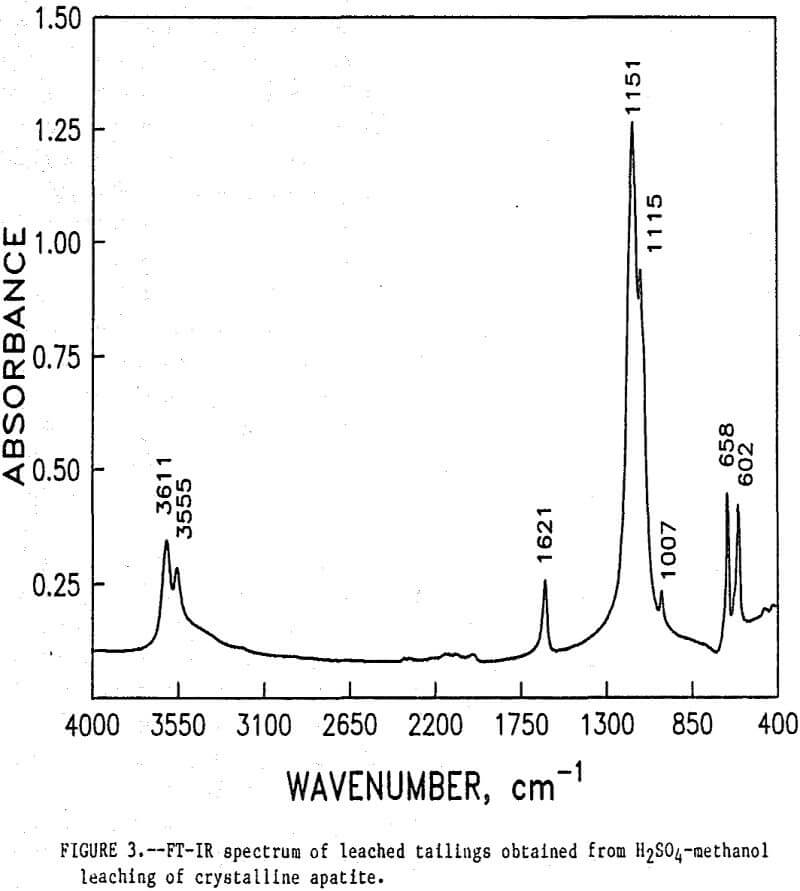
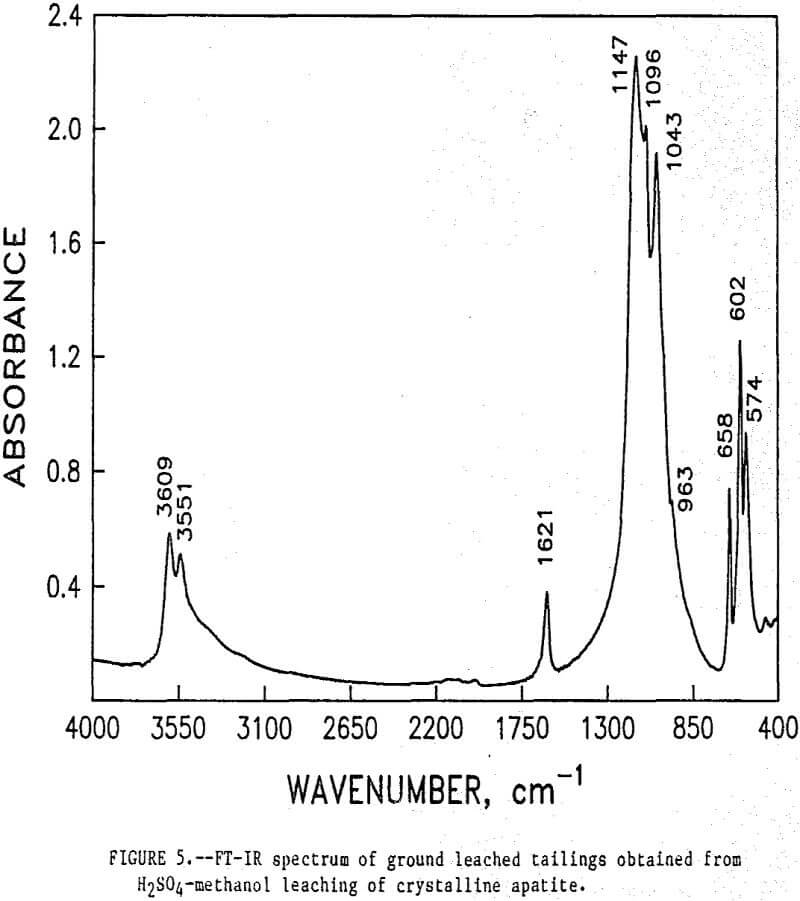
The initial identification of a coating layer was obtained by petrographic analysis of pure apatite before and after acid-alcohol leaching. Fluorapatite is characterized by the following indices of refraction: ω = 1.633; ε = 1.630. However, an oil of refractive index 1.636 proved the most effective in rendering the untreated apatite particles translucent. Figure 1 shows photographs taken on an optical microscope of pure apatite, sized at minus 28- plus 100-mesh, before and after acid-alcohol treatment. The translucent apatite particles have been darkened by a coating after leaching in the H2SO4-methanol system. To further substantiate the formation of a coating, an FT-IR spectrographic study was performed. The specific objective was to identify the coating and investigate whether its formation hindered further leaching. Figures 2 and 3 show the absorption spectra of the raw leached tailings (produced by leaching minus 28- plus 100-mesh apatite) and reagent-grade CaSO4·½H2O. Table 2 compares the peak wavenumbers for each sample.
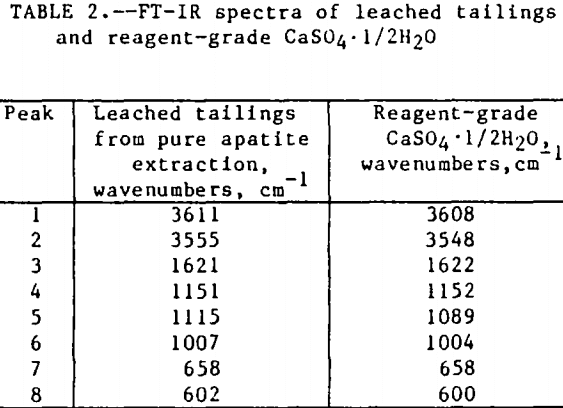
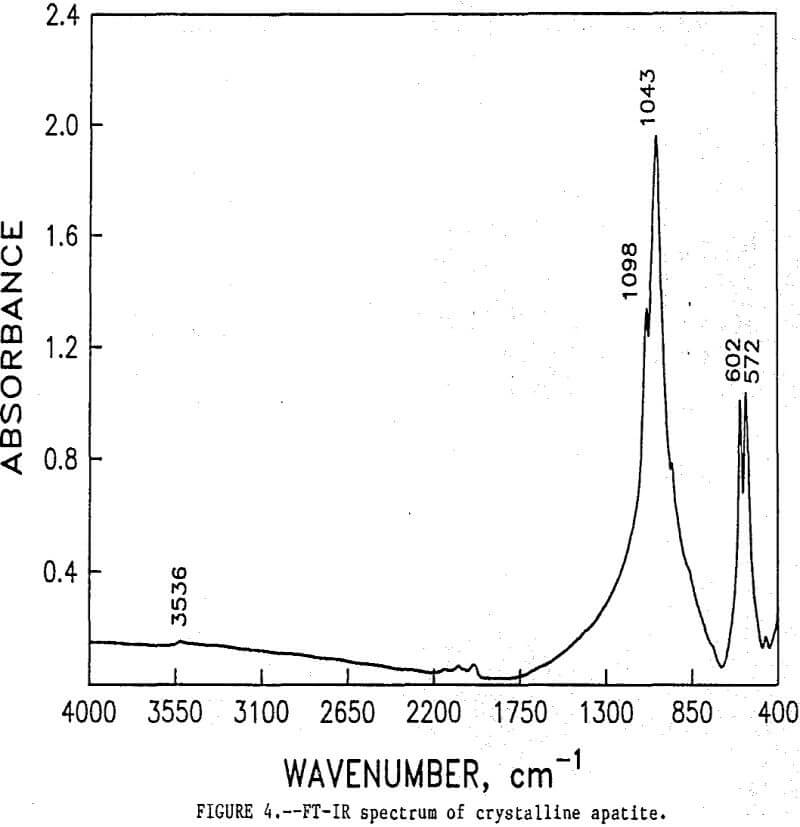
As shown, the two spectra match, peak for peak. XRD analyses of the leached tailings also indicate the hemihydrate form is the predominate byproduct of acid-alcohol leaching instead of gypsum. Figures 4 and 5 depict spectra of untreated apatite and leached tailings that have been ground to minus 325 mesh. A careful comparison of figures 3 and 5 shows that in the spectrum of the ground leached tailings, two additional absorption peaks are present at 1,043 cm-¹ and 574 cm-¹ which are not present in the spectrum of the raw tailings. Also, comparing the ground tailings spectrum (fig. 5) to the pure apatite spectrum (fig. 4), it is observed that these two additional peaks correspond to peaks found in the spectrum of pure apatite as shown in table 3.
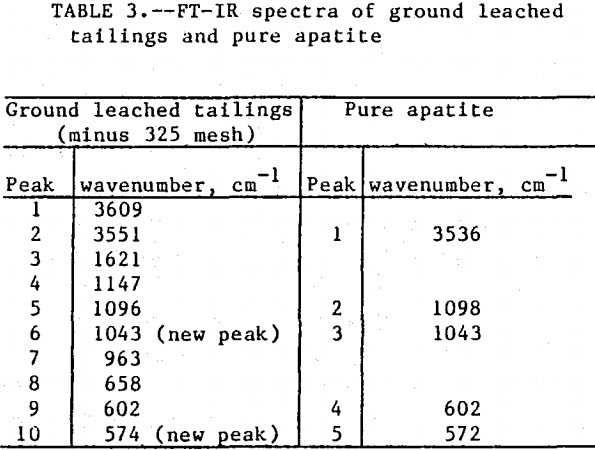
The spectra in figures 3, 4, and 5 strongly suggest that the acid-alcohol treated apatite is coated with CaSO4·½H2O and that infrared analysis did not detect the apatite underneath the coating layer. However, after grinding, some unleached apatite was exposed and detected by the FT-IR.
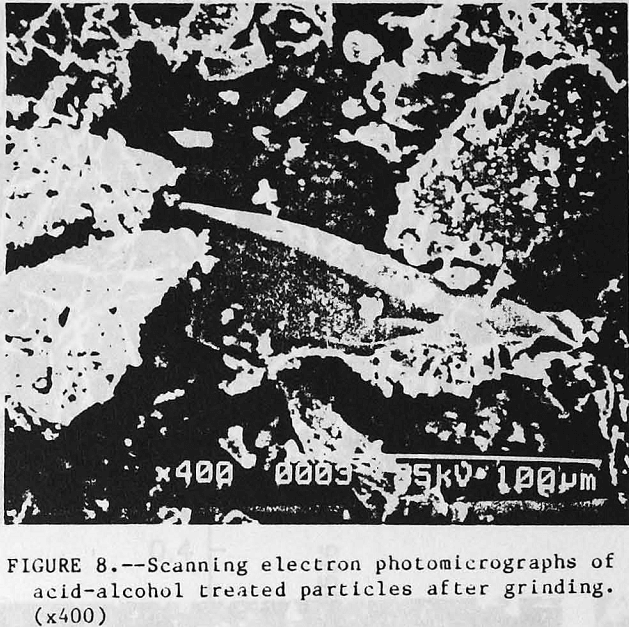
Additional visual evidence of particle coating was obtained using scanning electron microscopy (SEM). Profound changes were observed on the apatite surface after acid-alcohol leaching. Figure 6 (top) depicts the untreated apatite as having a smooth surface with sharp, well defined edges. After acid-alcohol leaching, the surface morphology is changed as shown in figure 6 (bottom); the hemihydrate coating gives a matted fiber appearance to the apatite particles, rounding-off the edges. A close-up of the barrier layer in figure 7 (top) vividly shows the long slender hemihydrate crystals. Figure 7 (bottom) depicts the pure apatite surface before treatment. Figure 8 portrays the leached apatite after grinding and clearly shows the coating has been removed and fresh apatite surface is exposed which is similar to the untreated particles shown in figure 6 (top).
Experimental extraction data also provided evidence indicating in situ coating formation. The objective here was to determine if a higher extraction level was possible by removing the barrier layer. Crystalline apatite, minus 28- plus 100-mesh, was slurried with methanol and acidulated with a stoichiometric amount of acid. The coating was identified on the tailing particles and the extent of phosphorus extraction was 18 pet. The tailings were then re-slurried in methanol and again treated with a stoichiometric amount of acid based on the amount of phosphorus remaining in the tailings. Only an additional 4 pct was extracted.
In another set of experiments, minus 28- plus 100-mesh apatite was leached under the same conditions. The phosphorus extraction level was 17 pct. The tailings were then ground to minus 100- plus 150-mesh and re-extracted with a stoichiometric amount of acid based on the amount of phosphate present. An additional 60 pct of the resident phosphate was extracted.
Thus, the overall extraction in the second case was around 67 pct. To determine whether the increased extraction resulted merely from the smaller particle size of ground leached tailings, a separate test was conducted in which research-grade apatite, ground to minus 100 plus 150 mesh, was leached under the same conditions. The extent of phosphate extraction obtained in this test was 32 pct. Clearly, the leach-grind-leach sequence produced a superior result.
Conclusions
The majority of phosphatic clay wastes leached using the H2SO4-methanol system produce recoveries in the 60- to 70-pct range leaving phosphate in the tailings and sulfuric acid in the leach liquors. Acid-alcohol leaching experiments using crystalline apatite facilitated the discovery of a calcium sulfate hemihydrate coating that forms in situ on the apatite particles. Formation of this coating on the apatite suggests a cause for the incomplete extractions of phosphate from the waste materials. Petrographic and FT-IR analyses were used to show that the CaSO4·½H2O barrier forms during leaching. Acid-alcohol leaching experiments using crystalline apatite have also shown that if the leached tailings are ground and are subsequently re-leached, an additional 60 pct of the phosphorus can be extracted.