A new suspension electrolysis method has been developed, which enables without any gas or dust pollutants a direct anodic extraction of zinc from zinc sulfide concentrates, while simultaneously oxidizing sulfide sulfur to elemental sulfur. The investigations were performed in a laboratory scale electrolytic cell with suspended zinc sulfide concentrate particles with or without graphite powder additions in dilute sulfuric acid electrolyte.
Experimental
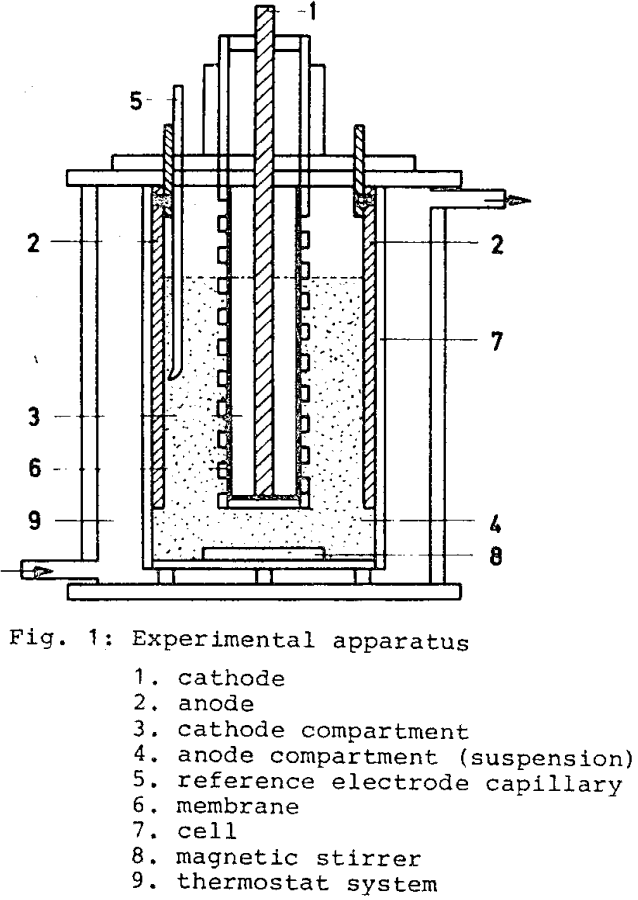
The cathode, a rod of aluminum, is placed in the center of cell. The anode consists of four plates mounted on the cell wall. The anode materials used in this investigations are PbO2(Pb), graphite and Pt. The cathode compartment and the anode compartment are separated by a “RCH 1000 Supralen-M-Type” membran, which prevents the transport of sulfide particles to the cathode area. With the capillary the reference electrode Hg/Hg2SO4 (sat. K2SO4) is connected with the system. To observe the suspension, which is obtained by a magnetic stirrer, the cell consists of transparent acryl glass. The temperature was kept constant by a thermostat in combination with circulating water in the double-walled cell.
With zinc blende particles suspended in 1 M H2SO4 solution a redox potential of about 0,15 V vs NHE could be observed. To avoid during the electrolysis of zinc concentrates the formation of H2S gas it is necessary to adjust electrolysis conditions which secure according to the Pourbaix-Diagramm, Fig. 2, that the anodic dissolution takes place in the potential range of the region, marked with the dissolution products Zn²+ and S°.
The results reveal that Pt has generally the highest oxygen overvoltage, followed by PbO2(Pb) electrode and graphite. The oxygen overvoltage decreases with increasing temperature. This tendency is not significant in the case of Pt and PbO2(Pb) but markedly to be seen in the case of graphite. Although Pt-electrodes allow the optimum electrolytic performance, the less expensive anode materials (PbO2) Pb and graphite were used in the course of the suspension electrolysis investigations.
Considerations
Adding zinc sulfide into 1 M H2SO4 solution H2S gas is generally generated by the following reaction:
ZnS + H2SO4 → ZnSO4 + H2S
The redox potential in this solution was estimated to approximately 0,146 – 0,156 V vs NHE. According to the potential pH diagram of ZnS-H2O system, this potential is located in the region of H2S generation. Adding graphite powder into the H2S generating solution, the H2S generation is reduced gradually and redox potential of the solution changes from 0,146 – 0,156 V to 0,416 – 0,426 V vs NHE. This redox potential is located in the area of S- respectively SO4- formation. Hereupon the potential of zincblende will be somewhat elevated, because graphite powder in the electrolyte has potential which is higher than zincblende as it can be seen from the potential-time curves
(O) + 2H+ + 2e → H2O
ZnS → Zn²+ + S° + 2e
If the potential of zincblende, which has to be considered as a mixed-potential under electrolysis conditions, is shifted by this galvanic reciprocal reaction into the region of S° formation the H2S generation will be suppressed. The H2S generation reaction is also suppressed by addition of Fe³+, MnO2 and KMnO4
Although the sulfate formation reaction should be expected thermodynamically due to the existing anode potential in this suspension electrolysis series, the reaction velocity of S° oxidation to SO4²- is so slow that elemental sulfur is still generated even at high potentials.