SCOPE AND APPLICATION
The method is designed to determine the concentration of total cyanide in aqueous/water and soil/sediment samples taken from hazardous waste sites.
SUMMARY OF METHOD
Aqueous/Water and Soil/Sediment
The cyanide as hydrocyanic acid (HCN) is released from cyanide complexes by means of a reflux-distillation, using either a midi- or micro-distillation process, and absorbed in a scrubber containing sodium hydroxide solution. The cyanide ion in the absorbing solution is then determined spectrophotometrically.
In the semiautomated spectrophotometric measurement, the cyanide is converted to cyanogen chloride (CNCl), by reaction with chloramine-T at a pH less than 8 without hydrolyzing to the cyanate. After the reaction is complete, color is formed on the addition of pyridine- barbituric acid reagent. The absorbance is read between 570 and 580 nanometers (nm). To obtain colors of comparable intensity, it is essential to have the same salt content in both the sample and the standards.
DEFINITIONS
See Exhibit G – Glossary of Terms for a complete list of definitions.
INTERFERENCES
Several interferences are encountered with this method. Some of the known interferences are aldehydes, nitrate-nitrite, oxidizing agents such as chlorine, thiocyanate, thiosulfate and sulfide. Some interferences are eliminated or reduced by using the distillation procedure. Some specific interferences that are commonly encountered are further discussed below.
Sulfides
Sulfides adversely affect the spectrophotometric procedure. The sample should be tested in the field for the presence of sulfides as described lower.
Surfactants
The presence of surfactants may cause the sample to foam during refluxing. If this occurs, the addition of an agent such as Dow Corning 544 antifoam agent, or equivalent, will prevent the foam from collecting in the condenser.
Oxidizing Agents
Oxidizing agents such as chlorine decompose most of the cyanides. The sample should be tested in the field for the presence of oxidizing agents as described lower.
Nitrates-Nitrites
High results may be obtained for samples that contain nitrate and/or nitrite. During the distillation, nitrate and nitrite will form nitrous acid that will react with some organic compounds to form oximes. These oximes will decompose under test conditions to generate HCN. The interference of nitrate and nitrite is eliminated by pretreatment with sulfamic acid prior to the distillation process.
SAFETY
See Introduction to Analytical Methods.
EQUIPMENT AND SUPPLIES
Brand names, suppliers, and part numbers are for illustrative purposes only. No endorsement is implied. Equivalent performance may be achieved using equipment and supplies other than those specified here; however, a demonstration of equivalent performance meeting the requirements of this Statement of Work (SOW) is the responsibility of the Contractor. The Contractor shall document any use of alternate equipment or supplies in the Sample Delivery Group (SDG) Narrative.
Midi-Distillation of Aqueous/Water and Soil/Sediment Samples
- Midi-reflux distillation apparatus
- Heating block – Capable of maintaining 125°C (±5°C)
- Auto analyzer system with accessories:
Sampler
Pump
Cyanide cartridge
Spectrophotometer with 50 mm flow cells and 580 nm filter
Chart recorder or data system - Assorted volumetric glassware, pipets, and micropipets (Class A)
- Top loader balance, 300 g capacity, and a minimum sensitivity of ±1.0 mg
Micro-Distillation of Aqueous/Water and Soil/Sediment Samples
- Micro-distillation apparatus
- Heating block – capable of maintaining 120°C (±5°C)
- Micro-distillation tubes – Sample tubes and Collector tubes, either pre-filled or user-filled with trapping solution
- Tube press
- Flow Injection Analyzer with accessories
- Spectrophotometer with 580 nm filters
- Assorted volumetric glassware, pipets, and micropipets (Class A)
- Top loader balance, 300 g capacity and sensitivity of ±1.0 mg
REAGENTS AND STANDARDS
Reagents
- Reagent water – The purity of this water must be equivalent to ASTM Type II water (ASTM D1193-06). Use this preparation for all reagents, standards, and dilutions of solutions.
- Lead acetate Test Paper.
- Cadmium carbonate (Powdered).
- Potassium Iodide – Starch Test Paper.
- Ascorbic acid (Crystals).
- Midi-Distillation and Preparation Reagents for Aqueous/Water and Soil/Sediment Samples
- Sodium hydroxide absorbing solution and sample wash solution, 0.25N – Dissolve 10.0 g sodium hydroxide in reagent water and dilute to 1 L.
- Sodium hydroxide solution, 1.25N – Dissolve 50.0 g sodium hydroxide in reagent water and dilute to 1 L.
- Sulfuric acid, 50% (v/v) – Carefully add a portion of concentrated sulfuric acid to an equal portion of reagent water.
- Magnesium chloride solution (2.5M) – Weigh 510 g of MgCl2•6H2O into a 1000 milliliter (ml) flask, dissolve, and dilute to 1 L with reagent water.
- Sulfamic acid (Powdered).
- Micro-Distillation and Preparation Reagents for Aqueous/Water and Soil/Sediment Samples
- Sodium hydroxide absorbing solution and sample wash solution, 0.25N – Dissolve 10.0 g sodium hydroxide in reagent water and dilute to 1 L.
- Sodium hydroxide solution, 1.25N – Dissolve 50.0 g sodium hydroxide in reagent water and dilute to 1 L.
- Sulfuric Acid/Magnesium Chloride solution (7.11 M sulfuric acid/0.79 M magnesium chloride) – In a fume hood, weigh 32.2 g MgCl2•6H2O into a tared 500 mL beaker and add 110.8 g reagent water. Add 139 g concentrated sulfuric acid in 40 g portions with stirring. Allow the solution to cool.
- Sulfamic acid (Powdered).
- Spectrophotometric Reagents for Midi- and Micro-Distillation of Aqueous/Water and Soil/Sediment Samples
- Chloramine-T solution (0.014M) – Dissolve 0.40 g of chloramine-T in reagent water and dilute to 100 mL. Prepare fresh daily.
- Acetate Buffer – Dissolve 410 g of NaC2H3O2 • 3H2O in 500 mL of reagent water. Add sufficient glacial acetic acid to adjust pH to 4.5 (approximately 500 mL). An equivalent phosphate buffer may be substituted for the acetate buffer.
- Pyridine-barbituric acid solution – Transfer 15 g of barbituric acid into a 1 Liter volumetric flask. Add about 100 mL of
reagent water and swirl the flask. Add 75 mL of pyridine and mix. Add 15 mL of concentrated hydrochloric acid and mix. Dilute to about 900 mL with reagent water and mix until the barbituric acid is dissolved. Dilute to 1 L with reagent water. Store at 4°C (±2°C).
Standards
Introduction
The Contractor must provide all standards, except as noted, to be used with this contract. These standards may be used only after they have been certified according to the procedure in Exhibit E, Section 8.0. The Contractor must be able to verify that the standards are certified. Manufacturer’s certificates of analysis must be retained by the Contractor and presented upon request.
Stock Standard Solutions
Stock cyanide solution, 1000 mg/L CN – Dissolve 2.51 g of potassium cyanide and 2.0 g potassium hydroxide in reagent water
and dilute 1 L. Standardize with 0.0192N silver nitrate. Standardization is not necessary if this standard is purchased as a certified solution.
Intermediate cyanide standard solution, 10 mg/L CN – Dilute 1.0 mL of stock cyanide solution plus 20 mL of 1.25N sodium hydroxide solution to 100 mL with reagent water. Prepare this solution at time of analysis.
Secondary Dilution Standards
Prepare secondary dilution standard solutions by diluting the appropriate volumes of the intermediate cyanide standard solution with 0.25N sodium hydroxide. The final concentration of sodium hydroxide in all standards should be 0.25N.
SAMPLE COLLECTION, PRESERVATION, AND STORAGE
Sample Collection and Preservation
Aqueous/Water Sample Preservation
Collection of total cyanide must be in polyethylene or glass containers. The sample must be tested for sulfides and oxidizing agents, and preserved by the sampler immediately upon sample collection. Place a drop of the sample on lead acetate test paper to detect the presence of sulfides. If sulfides are present (test strip turns black), the sample volume required for the cyanide determination should be increased by 25 milliliters (mL). The total volume of sample should then be treated with powdered cadmium carbonate. Yellow cadmium sulfide precipitates if the sample contains sulfide. Repeat this operation until a drop of the treated sample solution does not darken the lead acetate test paper. Filter the solution through dry filter paper into a dry beaker, and from the filtrate measure the sample to be used for analysis. Avoid a large excess of cadmium carbonate and a long contact time in order to minimize a loss by complexation or occlusion of cyanide on the precipitated material. If no sulfides are present, test for the presence of oxidizing agents by placing a drop of the sample on a strip of potassium iodide – starch test paper (KI – starch paper); a blue color indicates the need for treatment. Add ascorbic acid, a few crystals at a time, until a drop of sample produces no color on the indicator paper. Then add an additional 0.6 g of ascorbic acid for each liter of sample volume. Preserve the sample with sodium hydroxide to pH greater than or equal to 12 and maintain at 4°C (±2°C) until distillation.
Soil/Sediment Sample Preservation
Samples shall be kept at 4°C (±2°C) from the time of collection until distillation.
Procedure for Sample Storage and Disposal
Aqueous/water samples must be protected from light and refrigerated at 4°C (±2°C) from the time of receipt until 60 days after delivery of a complete, reconciled data package to the U.S. Environmental Protection Agency (USEPA). After distillation, soil/sediment samples may be stored at room temperature within the laboratory until 60 days after delivery of a complete, reconciled data package to USEPA. After 60 days, the samples may be disposed of in a manner that complies with all applicable regulations.
The samples must be stored in an atmosphere demonstrated to be free of all potential contaminants.
Samples, sample distillates, and standards must be stored separately.
Contract Required Holding Time
The maximum sample holding time for cyanide is 12 days from Validated Time of Sample Receipt (VTSR).
CALIBRATION AND STANDARDIZATION
Instrument Operating Parameters
Because of the difference between various makes and models of satisfactory instruments, no detailed operating instructions can be provided. Instead, the analyst should follow the instructions provided by the manufacturer of the particular instrument. The Method Detection Limit (MDL) and precision must be investigated and established for cyanide on that particular instrument. All measurements must be within the operational range of the instrument. It is the responsibility of the analyst to verify the instrument configuration and operating conditions used satisfy the analytical requirements and to maintain Quality Control (QC) data confirming instrument performance and analytical results.
General Procedure
The following general procedure applies to most semi-automated spectrophotometers. Set up the manifold and complete system per manufacturer’s instructions. Allow the spectrophotometer and recorder to warm up for at least 30 minutes prior to use. Establish a steady reagent baseline, feeding reagent water through the sample line and appropriate reagents through reagent lines. Adjust the baseline using the appropriate control on the spectrophotometer. Prepare a standard curve by plotting the instrument response (e.g., absorbance) of standard on the Y-axis vs. concentration of the standard (in µg/L) on the X-axis.
Spectrophotometric Instrument Calibration Procedure
Instruments shall be calibrated daily or once every 24 hours, each time the instrument is set up, or after Initial Calibration Verification (ICV) or Continuing Calibration Verification (CCV) failure. The instrument standardization date and time shall be included in the instrument output.
The date and time of preparation and analysis shall be given in the laboratory logs (preparation) or instrument output (analysis).
Calibration standards shall be prepared fresh with each calibration performed. Calibrate the instrument with at least six standards, one of which must be a blank standard and one shall be at or below the Contract Required Quantitation Limit (CRQL), in graduated amounts in the appropriate range. The calibration curve shall be calculated using linear regression by plotting the concentration of cyanide in the standard (in µg/L) on the X-axis versus the instrument response (e.g., Absorbance) on the Y-axis. A standard linear regression, a weighted linear regression (e.g., 1/concentration or 1/concentration²), or a linear regression with zero force calibration model can be used as appropriate. No other types of equations (e.g., quadratic) are to be used. The acceptance criteria for the calibration curve is a correlation coefficient greater than or equal to 0.995. Sample analysis shall not begin until this criteria and the criteria in Sections 9.3.4 and 9.3.5 have been met.
The calibration equation must be checked to establish the representativeness of the data that were used to produce the calibration equation. This check involves the re-fitting of the non-blank calibration data back to the calibration equation or the comparison of the calculated concentration of each of the standards against the expected concentration of the associated standard. This difference is related to the actual residual. For this calculation the Percent Difference shall be used where the above difference is further divided by the expected amount or concentration of the respective standard. If these Percent Differences for each of the standards do not fall within ±30%, then the calibration equation is not acceptable and must be corrected. If a standard is analyzed at a concentration that is below the CRQL and the above criteria is not met, that value can be excluded from the calibration curve as long as the lowest non-zero standard is still analyzed at or below the CRQL and all standards included in the calibration curve are continuous and consecutive.
The y-intercept from the linear regression initial calibration equation shall also be evaluated. If the y-intercept is not below the CRQL for the initial calibration curve, the calibration is not acceptable and must be corrected. Samples are not to be analyzed until the y-intercept for the initial calibration curve meets the acceptance criteria.
Any changes or corrections to the analytical system shall be followed by recalibration.
Initial Calibration Verification (ICV)
Immediately after each cyanide system has been calibrated, the accuracy of the initial calibration shall be verified and documented for cyanide by the analysis of the ICV Solution at the wavelength used for analysis.
The ICV Solution is obtained from USEPA. If the solution is not available, the ICV solution can be prepared by the laboratory using a certified solution of the analyte from an independent source. Analyses shall be conducted at a concentration other than that used for instrument calibration, but near the middle of the calibration range. An independent source is defined as a standard composed of the analyte from a different source than those used in the standards for the instrument calibration.
The ICV shall be processed in the same manner as the standards used for the initial calibration for the method used.
Continuing Calibration Verification (CCV)
To ensure calibration accuracy during each analytical run sequence, a CCV shall be analyzed and reported at a frequency not to exceed every 1 hour during an analytical run sequence. The standard shall also be analyzed and reported at the beginning of the analytical run sequence and after the last analytical sample. See the example sample analytical run sequence in Section 12.7. This CCV standard shall be prepared from the same source and at the same mid-level concentration as the standards used during the initial calibration.
The same CCV standard shall be used throughout the analytical run sequences for a Sample Delivery Group (SDG) of samples received.
Each CCV analyzed shall be processed in the same manner as the standards used for the initial calibration for the method used.
PROCEDURE
Sample Preparation
If insufficient sample amount (less than 90% of the required amount) is received to perform the analyses, the Contractor shall contact the Sample Management Office (SMO) to inform them of the problem. SMO will contact the USEPA Region for instructions. The USEPA Region will either require that no sample analyses be performed or will require that a reduced volume be used for the sample analysis. No other changes in the analyses will be permitted. The Contractor shall document the USEPA Region’s decision in the Sample Delivery Group (SDG) Narrative.
If multi-phase samples (e.g., two-phase liquid sample, oily sludge/sandy soil/sediment sample) are received by the Contractor, the Contractor shall contact SMO to apprise them of the type of sample received. SMO will contact the USEPA Region. If all phases of the sample are amenable to analysis, the USEPA Region may require the Contractor to do any of the following:
- Mix the sample and analyze an aliquot from the homogenized sample.
- Separate the phases of the sample, and analyze one or more of the phases separately. SMO will provide EPA sample numbers for the additional phases, if required.
- Do not analyze the sample.
If all of the phases are not amenable to analysis (i.e., outside scope), the USEPA Region may require the Contractor to do any of the following:
- Separate the phases and analyze the phase(s) that is (are) amenable to analysis. SMO will provide EPA sample numbers for the additional phases, if required.
- Do not analyze the sample.
No other changes in the analyses will be permitted. The Contractor shall document the USEPA Region’s decision in the SDG Narrative.
Before preparation is initiated for an aqueous/water sample, the Contractor shall test for the presence of sulfides and oxidizing agents (e.g., residual chlorine). The test for sulfides shall be performed by placing a drop of the sample on a strip of lead acetate paper. If the test strip turns black, the Contractor shall treat the total volume of sample with powdered cadmium carbonate. Yellow cadmium sulfide precipitates when the sample contains sulfide.
This operation shall be repeated until a drop of the treated sample solution does not darken the lead acetate test paper. The solution shall be filtered through dry filter paper into a dry beaker, and the volume of sample to be used for analysis shall be measured from the filtrate. It is recommended that the Contractor avoid a large excess of cadmium carbonate and a long contact time in order to minimize a loss by complexation or occlusion of cyanide on the precipitated material. The test for oxidizing agents shall be performed by placing a drop of the sample on a strip of potassium iodide – starch test paper (KI – starch paper). If the test strip turns blue, the Contractor shall contact SMO for further instructions from the USEPA Region before proceeding with sample preparation and analysis. The Contractor shall document the presence of sulfides or oxidizing agents in the SDG Narrative. The Contractor shall document the results (positive or negative) of the tests for sulfides and oxidizing agents in the distillation log.
Aqueous/Water and Soil/Sediment Preparation of Standards and Samples
Standards Preparation
All standards for the midi-distillation and micro-distillation semi-automated spectrophotometric analysis shall be distilled in the same manner as the samples.
Standards for Midi-Distillation Preparation and Semi-Automated Spectrophotometric Analysis of Aqueous/Water and Soil/Sediment Samples
Calibration standards – Prepare a minimum of five standards and a calibration blank over the range of the analysis. These standards shall be prepared by pipetting suitable volumes of the secondary dilution standard solution into volumetric flasks and diluting to volume with 0.25N sodium hydroxide. Add 50 mL of each standard to a midi-distillation tube and then prepare and distill these standards and the calibration blank in the same manner as the samples.
NOTE: The concentration of one of the calibration standards shall be at or below the Contract Required Quantitation Limit (CRQL).
Standards for Micro-Distillation Preparation and Semi-Automated Spectrophotometric Analysis of Aqueous/Water and Soil/Sediment Samples
Calibration Standards – Prepare a minimum of five standards and a calibration blank over the range of the analysis. These standards shall be prepared by pipetting suitable volumes of the secondary dilution standard solution into volumetric flasks and diluting to volume with 0.25N sodium hydroxide. Add 6 mL of each standard to a sample tube and then prepare and distill these standards and the calibration blank in the same manner as samples.
NOTE: The concentration of one of the calibration standards shall be at or below the CRQL.
Aqueous/Water Samples Preparation
Preparation Method for Aqueous/Water Samples by Midi-Distillation
The procedure described here utilizes a midi-distillation apparatus and requires a sample aliquot of 50 mL for aqueous/water samples. The sample shall not be diluted prior to distillation.
Pipet 50 mL (±1 mL) of sample into the distillation flask along with 2 or 3 boiling chips.
Add 50 mL (±1 mL) of 0.25N sodium hydroxide to the gas absorbing tube.
Connect the boiling flask, condenser, and absorber in the train. The excess cyanide trap contains 0.5N sodium hydroxide.
Turn on the vacuum and adjust the gang (Whitney) valves to give a flow of between 2 to 3 bubbles per second from the impingers in each reaction vessel.
If the samples contain nitrate and/or nitrite, add 0.2 g of sulfamic acid through the air inlet tube. Mix for 3 minutes prior to adding the sulfuric acid.
After 5 minutes of vacuum flow, inject 5 mL of 50% (v/v) sulfuric acid through the top air inlet tube of the distillation head into the reaction vessel. Allow the airflow to mix the reaction vessel contents for 5 minutes.
NOTE: The acid volume must be sufficient to bring the sample/solution pH to below 2.0.
Add 2 mL of the 2.5M magnesium chloride solution through the top air inlet tube of the distillation head into the reaction vessel. Excessive foaming from samples containing surfactants may be quelled by the addition of either another 2 mL of the
2.5M magnesium chloride solution or a few drops of a commercially available anti-foam agent. The Contractor shall document the addition of magnesium chloride solution or antifoam agent in the SDG Narrative.
Turn on the heating block and set for 125°C (±3°C). Heat the solution to boiling, taking care to prevent solution backup by periodic adjustment of the vacuum flow.
After 1½ hours of refluxing, turn off the heat and continue the vacuum for an additional 15 minutes. The flasks should be cool at this time.
After cooling, close off the vacuum at the gang valve and remove the absorber. Seal the receiving solutions and store them at 4°C until analyzed.
Preparation Method for Aqueous/Water Samples by Micro-Distillation
Preheat the heater block to 120°C (±3°C). Add 6 mL (±0.1 mL) of sample to the sample tube. The sample shall not be diluted prior to distillation. If the Contractor is not using the prefilled collector tubes, add 2 mL (±0.1 mL) of the 0.25N absorbing solution to each collector tube. Add 0.75 mL of the (7.11M/0.79M) sulfuric acid/magnesium chloride solution to each sample tube and immediately cap with a collector tube and press to seal.
Place the assembled tubes into the heater block and heat for 30 minutes. After 30 minutes, remove each tube from the block
and immediately pull off the sample tube.
Invert each collector tube and allow to cool. Mix the distillate and detach the upper portion. Dilute the distillate to 6 mL (±0.1 mL) with absorbing solution and mix. The distillate is now ready for analysis. Seal the distillate and store at 4°C until analyzed.
Soil/Sediment Samples Preparation
Preparation Method for Soil/Sediment Samples by Midi-Distillation
The procedure described here utilizes a midi-distillation apparatus and requires a sample aliquot of at least 1 g for soil/sediment.
Mix the sample thoroughly to achieve homogeneity (see Exhibit D – Introduction to Analytical Methods, Section 1.8). For each digestion procedure, weigh to the nearest 0.01 g and transfer 1.00 – 1.50 g of sample (wet weight) into the reaction vessel and add 50 mL of reagent water. Add 2 or 3 boiling chips.
Add 50 mL (±1 mL) of 0.25N sodium hydroxide to the gas absorbing impinger.
Connect the reaction vessel, condenser, and absorber in the train. The excess cyanide trap contains 0.5N sodium hydroxide.
Turn on the vacuum and adjust the gang (Whitney) valves to give a flow of between 2 to 3 bubbles per second from the impingers in each reaction vessel.
After 5 minutes of vacuum flow, inject 5 mL of 50% (v/v) sulfuric acid through the top air inlet tube of the distillation head into the reaction vessel. Allow to mix for 5 minutes.
NOTE: The acid volume must be sufficient to bring the sample/solution pH to below 2.0.
Add 2 mL of the 2.5M magnesium chloride solution through the top air inlet tube of the distillation head into the reaction vessel. Excessive foaming from samples containing surfactants may be quelled by the addition of either another 2 mL of the
2.5M magnesium chloride solution or a few drops of a commercially available anti-foam agent. The Contractor shall document the addition of the 2.5M magnesium chloride solution or anti-foam agent in the SDG Narrative.
Turn on the heating block and set for 125°C (±3°C). Heat the solution to boiling, taking care to prevent solution backup by periodic adjustment of the vacuum flow.
After 1½ hours of refluxing, turn off the heat and continue the vacuum for an additional 15 minutes. The flasks should be cool at this time.
After cooling, close off the vacuum at the gang valve and remove the absorber. Seal the receiving solutions and store them at 4°C until analyzed.
Preparation Method for Soil/Sediment Samples by Micro-Distillation
Preheat the heater block to 120°C (±3°C). Add 0.50-1.00 g (±0.01 g) of sample (wet weight) and 5 mL of reagent water to the sample tube. If the Contractor is not using the prefilled collector tubes, add 2 mL (±0.1 mL) of the 0.25N absorbing solution to the collector tube. Add 0.75 mL of the (7.11M/0.79M) sulfuric acid/magnesium chloride solution to each sample tube and immediately cap with collector tube and press to seal.
Place the assembled tubes into the heater block and heat for 30 minutes. After 30 minutes, remove each tube from the block and immediately pull off the sample tube.
Invert each collector tube and allow to cool. Mix the distillate and detach the upper portion. Dilute the distillate to 6 mL (±0.1 mL) with absorbing solution and mix. The distillate is now ready for analysis. Seal the distillate and store at 4°C until analyzed.
Sample Analysis
Semi-Automated Spectrophotometric Determination of Distillates
Set up the manifold. Pump the reagents through the system until a steady baseline is obtained.
Place the distilled calibration standards, blanks, and control standards in the sampler tray, followed by the distilled samples, duplicates, standards, spikes, and blanks. Allow all standards and samples to come to ambient room temperature prior to analysis.
When a steady reagent baseline is obtained and before starting the sampler, adjust the baseline using the appropriate knob on the spectrophotometer. Aspirate the distilled blank calibration standard and adjust the spectrophotometer until the desired signal is obtained. Establish the baseline and proceed to analyze the remainder of the distilled standards and distilled samples.
Sample distillates having concentrations higher than the established calibration range as determined by the expected concentration of the highest calibration standard shall be diluted into range with the absorbing solution and reanalyzed.
DATA ANALYSIS AND CALCULATIONS
Calculations for Midi- and Micro-Distillation of Aqueous/Water and Soil/Sediment Samples
Calculations for Semi-automated Spectrophotometric Determination
Prepare a standard curve by plotting the instrument response of the standards against their true concentrations in µg/L using linear regression as discussed lower. This standard curve is then used to determine the concentration of cyanide in
the field and Quality Control (QC) samples.
Calculate the cyanide concentration in aqueous/water samples by the formula:
EQ. 1 Aqueous/Water Sample Concentration
CN Concentration (ug/L) = C x Vf/V x DF
WHERE, C = Instrument response in μg/L CN from the calibration curve
Vf = Final prepared (absorbing solution) volume (mL)
V = Initial aliquot amount (mL)
DF = Dilution Factor
Calculate the cyanide concentration in soil/sediment samples by the formula:
EQ. 2 Soil/Sediment Sample Concentration
CN Concentration (mg/kg) = C x Vf/W x S x (1/1000) x DF
WHERE, C = Instrument response in μg/L CN from the calibration curve
Vf = Final prepared (absorbing solution) volume (mL)
W = Initial aliquot amount (g)
S = % Solids/100 (see Exhibit D – Introduction to Analytical Methods)
DF = Dilution Factor
Adjusted Method Detection Limit (MDL)/Adjusted Contract Required Quantitation Limit (CRQL) Calculation
To calculate the adjusted aqueous/water MDL or CRQL for the midi and micro methods, multiply the MDL or CRQL (µg/L) by the sample Dilution Factor.
The adjusted soil/sediment MDL or CRQL for the midi- and micro-distillation methods shall be calculated as follows:
EQ. 3 Adjusted Soil/Sediment MDL or CRQL
Adjusted Concentration (mg/kg) = C x Wm/W x S x DF
WHERE, C = MDL or CRQL (mg/kg)
Wm = Minimum method required aliquot amount (1.00 g for midi or 0.50 g for micro)
W = Initial aliquot amount (g)
S = % Solids/100 (see Exhibit D – Introduction to Analytical Methods)
DF = Dilution Factor
QUALITY CONTROL (QC)
Initial Calibration Verification (ICV)
The ICV standard shall be prepared in the same matrix as the calibration standards and in accordance with the instructions provided by the supplier. The ICV standard shall be processed in the same manner as the standards used for the initial calibration for the method used. If measurements exceed the control limits of 85% Recovery (low) or 115% Recovery (high), the analysis shall be terminated, the problem corrected, the instrument recalibrated, and the calibration reverified. The results of the ICV analysis shall be reported.
Continuing Calibration Verification (CCV)
The CCV standard shall be prepared in the same matrix as the calibration standards by the analyst at a concentration equivalent to the mid-point of the calibration curve using the same standards that were used to prepare the initial calibration curve. The CCV standard shall be processed in the same manner as the standards used for the initial calibration for the method used. If the measurement exceeds the control limits of 85% Recovery (low) or 115% Recovery (high), the analysis shall be stopped, the problem corrected, the instrument recalibrated, the calibration verified, and reanalysis of all analytical samples analyzed since the last compliant calibration verification shall be performed. The results of all CCV analyses shall be reported.
Blank Analyses
There are two different types of blanks required by this method. The calibration blank is used in establishing the analytical curve. The Initial and Continuing Calibration Blanks (ICB/CCB) are identical in composition to the calibration blank and are analyzed immediately after the ICV/CCV to monitor for potential carryover of the analyte. The Preparation Blank is used to monitor for possible contamination throughout the entire sample preparation and analysis process.
Initial and Continuing Calibration Blank (ICB/CCB)
The ICB and CCB are identical in composition to the Calibration Blank as used to establish the initial calibration curve. The ICB/CCB shall be processed in the same manner as the standards used for the initial calibration for the method used. If the absolute value of the calibration blank (ICB/CCB) result exceeds the Contract Required Quantitation Limit (CRQL) (see Exhibit C), the analysis shall be terminated, the problem corrected, the instrument recalibrated, the calibration verified, and reanalysis of all analytical samples analyzed since the last compliant calibration blank shall be performed. Results of the ICB and CCB analyses shall be reported.
Preparation Blank
The Preparation Blank shall contain all the reagents and in the same volumes as used in processing the samples. The Preparation Blank shall be carried through the complete preparation, distillation, and analysis method process.
At least one Preparation Blank, consisting of reagent water processed through each sample preparation and analysis procedure (see Section 10), shall be prepared and analyzed with every Sample Delivery Group (SDG), or with each batch of samples distilled, whichever is more frequent.
The first batch of samples in an SDG is to be assigned to Preparation Blank one, the second batch of samples to Preparation Blank two, etc. Each Complete SDG File (CSF) shall contain the results of all the Preparation Blank analyses associated with the samples in that SDG.
The Preparation Blank(s) is (are) to be reported for each SDG and used in all analyses to ascertain whether sample concentrations reflect contamination in the following manner:
- If the absolute value of the concentration of the blank is less than or equal to the CRQL (see Exhibit C), no further action is required.
- If the analyte concentration in the blank is above the CRQL, the lowest concentration of the analyte in the associated samples (except those identified as field blanks) shall be greater than or equal to 10 times the blank concentration. Otherwise, all samples (except those identified as field blanks) associated with the blank, with the analyte
concentration less than 10 times the blank concentration and above the CRQL, shall be redistilled and reanalyzed with appropriate new QC. The sample concentration is not to be corrected for the blank value. - If the concentration of the blank is below the negative CRQL, then all samples associated with the blank and reported below 10 times CRQL shall be reprepared and reanalyzed with appropriate new QC.
- The results for the Preparation Blank shall be reported.
Spike Sample Analysis
The spike sample analysis is designed to provide information about the effect of the sample matrix on the distillation and/or
measurement methodology. The spike is added before the distillation step and prior to the addition of other reagents. At least one spike sample analysis (matrix spike) shall be performed on each group of samples of a similar matrix type (i.e., aqueous/water, soil/sediment) or for each SDG, whichever is more frequent. The sample and its associated spike sample shall initially be run at the same dilution.
If the spike analysis is performed on the same sample that is chosen for the duplicate sample analysis, spike calculations shall be performed using the results of the sample designated as the “original sample”. The average of the duplicate results cannot be used for the purpose of determining percent recovery. Samples identified as field blanks and Performance Evaluation (PE) samples shall not be used for spiked sample analysis. USEPA may require that a specific sample be used for the spike sample analysis.
The analyte spike shall be added to achieve a concentration of 100 µg/L in the final sample solution prepared for analysis (i.e., post-distillation). For example, the midi-distillation procedure would require the addition of 5 µg of cyanide to the sample prior to distillation (based on the final distillate volume of 50 mL). For a typical 50 mL aqueous/water sample, this would be equivalent to a concentration of 100 µg/L in the original sample. For a typical 1.0 g soil/sediment sample, this would be equivalent to a concentration of 5 mg/kg in the original dry sample. Adjustments shall be made to maintain these spiking levels when the weight of the sample taken deviates by more than 10% of these values.
If the spike recovery is not at or within the limits of 75-125%, the data for all samples received and associated with that spike sample and determined by the same analytical method shall be flagged with the letter “N”. An exception to this rule is granted when the sample concentration exceeds the Spike Added (SA) concentration by a factor of four or more. In such an event, the data shall be reported unflagged even if the percent recovery does not meet the 75-125% recovery criteria.
When the matrix spike recovery falls outside the control limits and the sample result does not exceed four times the spike added, a post-distillation spike shall be performed. Note that if a post¬distillation spike analysis is required, the same USEPA sample that was used for the matrix spike analysis shall be used for the post¬digestion spike analysis. Spike the unspiked aliquot of the undiluted distillate at two times the indigenous level or two times the CRQL, whichever is greater.
In the instance where there is more than one spike sample per matrix, per method, per SDG, if one spike sample recovery is not within contract criteria, flag all the samples of the same matrix and method in the SDG. Individual component percent recoveries are calculated as follows:
EQ. 4 Matrix Spike and Post-Digestion Spike Percent Recovery
% Recovery = SSR – SR/SA x 100
WHERE, SSR = Spiked Sample Result (µg/L or mg/kg) from EQ. 1 or EQ. 2
SR = Sample Result (µg/L or mg/kg) from EQ. 1 or EQ. 2
SA = Spike Added Theoretical Result (µg/L or mg/kg).
This is calculated by substituting the spiking amount used for the ‘Vf’ term and substituting the spiking standard concentration used for the ‘C’ term from EQ. 1 or EQ.2.
When the sample concentration is less than the Method Detection Limit (MDL), use SR = 0 only for purposes of calculating the percent recovery. The Spike Sample Results (SSRs), Sample Results (SRs), Spike Added (SA), and percent recovery (positive or negative) shall be reported.
The units used for reporting Spike Sample Results (SSRs) will be identical to those used for reporting Sample Results (SRs).
Duplicate Sample Analysis
One duplicate sample shall be analyzed from each group of samples of a similar matrix type (i.e., aqueous/water, soil/sediment) or for each SDG, whichever is more frequent. Duplicates cannot be averaged for reporting.
Duplicate sample analyses are not required for percent solids. Samples identified as field blanks and PE samples shall not be used for duplicate sample analysis. USEPA may require that a specific sample be used for duplicate sample analysis. The Relative Percent Difference (RPD) is calculated as follows:
EQ. 5 Duplicate Sample Relative Percent Difference
RPD = S – D/(S + D)/2 x 100
WHERE, RPD = Relative Percent Difference
S = Sample Result (original) (µg/L or mg/kg) from EQ. 1 or EQ. 2
D = Duplicate Sample Result (µg/L or mg/kg) from EQ. 1 or EQ. 2
The results of the duplicate sample analyses shall be reported. A control limit of 20 for RPD shall be used for original and duplicate sample values greater than or equal to five times the CRQL (see Exhibit C). A control limit equal to the CRQL value shall be entered in the “Control Limit” column if either the sample or duplicate value is less than five times the CRQL. If the sample and duplicate values are greater than or equal to five times the CRQL, or if the sample and duplicate values are less than the CRQL, the “Control Limit” field is left empty.
If one result is above five times the CRQL level and the other is below, use the CRQL criteria to determine if the duplicate analysis is in control. If both sample and duplicate values are less than the CRQL, the RPD is not calculated. For soil/sediment sample or soil/sediment duplicate results less than five times the CRQL, enter the value of the CRQL, corrected for sample weight and percent solids, (i.e., original, not duplicate sample weight), in the “Control Limit” column. If the duplicate sample results are outside the control limits, flag all the data for samples received and associated with that duplicate sample with an. In the instance where there is more than one duplicate sample per SDG, if one duplicate result is not within contract criteria, flag all samples of the same matrix and method in the SDG. The percent difference data will be used by USEPA to evaluate the long-term precision of the method. Specific control limits for this analyte will be added at a later date based on the precision results.
MDL Determination
Before any field samples are analyzed under this contract, the MDLs shall be determined for each distillation procedure and instrument used, prior to the start of contract analyses, and annually thereafter. The MDLs shall meet the levels specified in Exhibit C. An MDL study shall be performed after major instrument maintenance, or changes in instrumentation or instrumental conditions, to verify the current sensitivity of the analysis.
To determine the MDLs, the Contractor shall run MDL studies following the procedures given in 40 CFR, Part 136. The
Contractor shall prepare the MDL samples by each digestion procedure used and shall analyze these samples on each instrument used.
The determined concentration of the MDL shall be less than half the concentration of the CRQL listed in Exhibit C.
The results of the MDL determination studies shall be forwarded to the USEPA Regional CLP PO, Sample Management Office (SMO), and Quality Assurance Technical Support (QATS).
The MDL results shall be reported on Form IX-IN.
Example Analytical Sequence for Cyanide
S0
S10
S50
S100
S200
S400
ICV
ICB
CCV
CCB
samples
CCV
CCB
samples
CCV
CCB, etc.
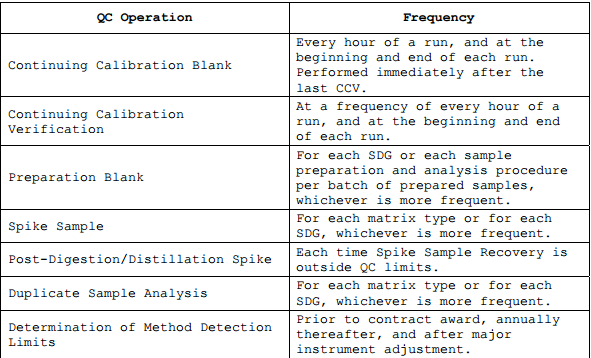
Summary of QC Operations
The QC operations performed for cyanide analysis are summarised in the table below.