Apparatus, Reagents,—The usual volumetric and gravimetric apparatus. For analysis the student may take the bench reagent labelled 5E. NH4HO. For the standard acid pure H2SO4 of specific gravity 1.840 or thereabouts is most suitable. Pure Na2CO3 (prepared as before) is necessary for standardising the acid.
Method, Reactions.—A certain volume of the ammonia solution is taken at a temptrature of 16° C. (approximately). After adding the indicator, the solution is titrated with a standard solution of H2SO4.
2NH4HO + H2SO4 = (NH4)2SO4 + 2H2O
The value and volume of the H2SO4 being known, the strength of the ammonia solution is easily calculated.
Preparation of the Standard Solution.—A normal solution of H2SO4 contains 2 + 32 + 64/2 = 49 gms. H2SO4 per litre. For present purposes the student may prepare a N/10 solution as follows:—
Measure out 3 c.cs. H2SO4 (S.G. 1.84). This quantity weighs about 5.5 gms. To this add about 100 c.cs. distilled water, and when cool transfer to the test-mixer and make up to 1000 c.cs. at about 16° C.
Checking and adjusting the Standard.—Measure out from the burette 4 samples of 20 c.cs. each. Fill a burette with N/10 Na2CO3. Add to each of the first two samples two drops of methyl orange and titrate with the N/10 Na2CO3. Note the readings. Dilute each of the other two samples (as an additional check) to 100 c.cs. with distilled water, and in each of them, estimate the SO3 by precipitation with BaCl2, (see Gravimetric Analysis), using preferably a Gooch crucible for filtration.
As an example, assume that the following results are obtained.
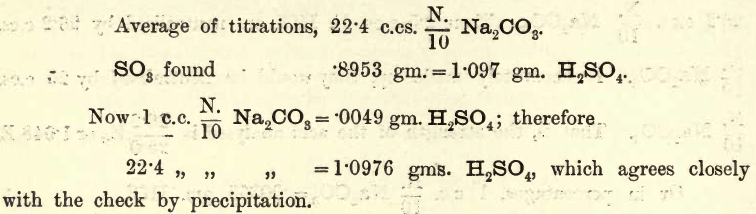
But 20 c.cs. N/10 H2SO4 should contain 20 x .0049 = 0.98 gm. H2SO4; that is, the acid is too strong in the proportion of 1.097 : .980. In practice, the chemist adopts one of two courses :—
1. A factor is calculated, and is then used in all subsequent determinations.
2. The acid is adjusted by the addition of water and rechecking.
Both methods give equally accurate results; the first method, being much more rapid, is better suited to the busy chemist. The factor is calculated thus: If the acid were exactly N/10, 20 c.cs. would require 20 c.cs. N/10 Na2CO3 for neutralisation; but 22.4 c.cs. were required, therefore 22.4/20 = 1.12 is the
required factor, and all readings of titrations with this acid must be multiplied by this factor.
If the second course is adopted the strength is adjusted as follows: Every 20 cc.s. of the acid must be diluted to 22.4 c.cs. Measure the volume of acid and dilute in this proportion. Titrate as before with N/10 Na2CO3 and readjust if not quite accurate. The student will find this process rather troublesome, more especially if he over dilutes the solution of acid. If in the least doubt, he should keep the acid a little on the strong side, as it is much easier to add water than acid.
Having standardised the acid by either of these methods, the student may proceed to the actual analysis.
Details of the Analysis.—From the reagent bottle, at a temperature of about 16° C., carefully measure by the burette 20 c.cs. of the 5 E. NH4HO. Dilute with distilled water to 500 c.cs. After thorough mixing, measure out two portions of 20 c.cs. each and to each portion add about 1 c.c. neutral litmus solution.
Titrate each portion with the N/10 H2SO4 till neutral, as shown by the indicator. The duplicates, as usual, should agree. Repeat the operation (as a check on the sampling) with a fresh sample from the reagent bottle.
Calculations.—Assume, for example, that the average reading is 38.8 c.cs. N/10 H2SO4. The volume of 5E. NH4HO corresponding to this is 20 x 20/500 = .8 c.c. If correctly made up, .8 c.c. 5E. NH4HO should require .8 x 5 x 10 = 40 c.cs. N/10 H2SO4. Therefore the real strength of the ammonia is 38.8/40 5 E. or .97 x 5E. = 4.85 E. Supposing the student is using the unadjusted standard solution and factor, the figures will be somewhat as follows:—
Average reading will be 34.6 c.cs. H2SO4 (N/10 unadjusted), multiplying by the factor 34.6 x 1.12 = 38.752, or to the nearest decimal in the first place, 38.8 c.cs. N/10 H2SO4. The remainder of the calculation is the same as the preceding. The student must remember that the factor 1.12 was assumed merely as an example, and that this factor, if used, must be accurately determined.
