The United States depends on imports of bauxite and alumina for most of its aluminum requirements. To reduce this dependence, the Bureau of Mines conceived a raw materials-process technologies matrix as a means of systematically investigating the technology options available for processing domestic aluminum resources. In 1973, a research program was initiated based on this matrix. Resources considered were clay, anorthosite, alunite, dawsonite contained in oil shale, and coal ash and coal shale.
Anorthosite and clay are major domestic resources that could meet our aluminum requirements indefinitely. Major deposits exist throughout the United States. Investigators have examined a number of techniques to process these deposits, but because of economic and environmental considerations, none of the processes has been commercialized.
Most of the research on alumina recovery from anorthosite was concentrated on the lime-soda-sinter process. Anorthosite is sintered with soda ash and limestone. Leaching, desilication, and precipitation steps are performed before alumina is recovered as the trihydrate. The lime-soda-sinter process has two major drawbacks;
- In addition to mining anorthosite, large amounts of limestone and soda ash are required,
- Sintering is performed at 1,300° C, which consumes appreciable amounts of energy.
Other disadvantages are the potential gelation of the leaching slurry, the relatively low alumina recovery (80 pct) and the generation of large quantities of waste products.
Another method for recovering alumina from anorthosite is the melt-quench technique, in which a charge of anorthosite is arc melted at approximately 1,650° C and then rapidly cooled. The resulting amorphous product is amenable to alumina extraction by acid.
Because of their crystalline structure, aluminum silicates, such as anorthosite, are not readily attacked by a mineral acid unless the crystalline structure is altered. Acid in conjunction with the fluoride ion disrupts the silicate structure sufficiently so that acid leaching is effective in extracting the aluminum content.
The use of a fluoride compound to increase aluminum extraction from silicates is an old concept. An English patent dated 1905 proposes the use of boiling hydrofluosilicic and sulfuric acids “for the recovery of the potash and alumina or other valuable constituents.” Two other patents issued in 1924 and 1928 mention the addition of a fluoride compound to the leaching acid. One patent proposes CaF2 addition to the hot sulfuric acid leach, while the other patent prefers an HCl-H2SiF6 acid leaching system. The latter patent claims that the fluoride addition is a substitute for calcination of the clay.
This study was undertaken to determine the feasibility of leaching anorthosite with acid and fluoride as an alternative to extractive techniques requiring high-temperature calcination. Three leaching modes, single-stage, cocurrent, and countercurrent leaching, were studied. The most efficient technique, countercurrent leaching, was examined extensively.
Materials
Anorthosite is a rock composed mainly of calcium-rich plagioclase. It is intermediate in composition between albite (NaAlSi3O8) and anorthite (CaAl2Si2O8). Anorthosite resources of the United States are estimated at 599 billion tons averaging 27 pct A12O3. Large anorthosite deposits are located in Minnesota, New York, Wyoming, and California.
The anorthosite used was taken from a quarry (Sec. 12, T 16 N, R 72 W) in the Laramie mountain range of Wyoming. A 38-lb sample was pulverized to minus 100 mesh, blended, and sampled. Chemical analysis of the anorthosite is presented in table 1. The fluoride compounds and HCl used were reagent grade.
Equipment
Leaching tests were performed in a 500-ml glass kettle, fitted with a sealed, ground glass cover. A glass stirring rod with a Teflon paddle was used for slurry agitation. The kettle vent was attached to a water-cooled condenser to reflux acid, which was returned to the kettle. Heat was supplied by an electric heating mantle.
Procedures
Most leaching tests used 50 grams of anorthosite. Occasionally, 150-gram samples were used in order to produce sufficient quantities of residue and pregnant liquor for more complete analysis.
A sample was placed in the kettle and the vessel sealed. Measured quantities of HCl and fluoride compound were added through a cover hole. The slurry was heated to 104° C. When this temperature was attained, the leaching cycle was started. At the termination of the experiment, the hot pulp was filtered on a glass-fiber filter paper. The leached residue in the filtration funnel was washed with three portions of water. Solutions were analyzed by wet-chemical analysis for aluminum. Aluminum values in the residue were determined by neutron activation. Experimental details pertinent to each leaching mode will be described in subsequent sections of the paper.
Results and Discussion
Single-Stage Leaching
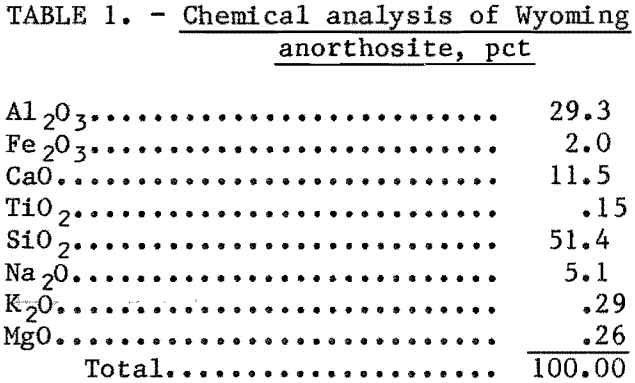
Quantity and concentration of the leaching acid were studied to determine the effect on aluminum recovery. Tests were made with and without the addition of fluoride. The results are summarized in figure 1. Hydrofluosilicic acid was added so that the ratio of fluoride in the leachant to aluminum in the ore was constant at 0.27 mole fluoride per mole of aluminum (F/Al = 0.27). Fifty-gram samples of anorthosite were leached with 300 to 400 ml of solution containing 7 to 36 pct HCl. Duration of leach was 4 hr.
The percent stoichiometric HCl on the abscissa of figure 1 refers to the amount of HCl theoretically required to react with all of the aluminum contained in the sample. Since calcium and the other constituents in anorthosite also consume HCl, acid consumption will be increased. If all acid-soluble constituents were completely leached, 140 pct of the HCl stoichiometrically required for aluminum extraction would be consumed.
Figure 1 shows that up to 50 pct of the aluminum can be leached with HCl alone if enough acid (approximately 300 pct of theoretical) is used. Addition of fluoride aluminum extraction up to 80 pct, but 400 pct of stoichiometric acid addition is required.
Acid concentration did not appreciably affect aluminum extraction. The important parameter was the quantity used rather than concentration. In subsequent tests, 20-pct HCl, the azeotropic concentration, was used because at stronger acid concentration, some HCl was lost through the condenser.
The effect of fluoride addition on aluminum extraction was determined.
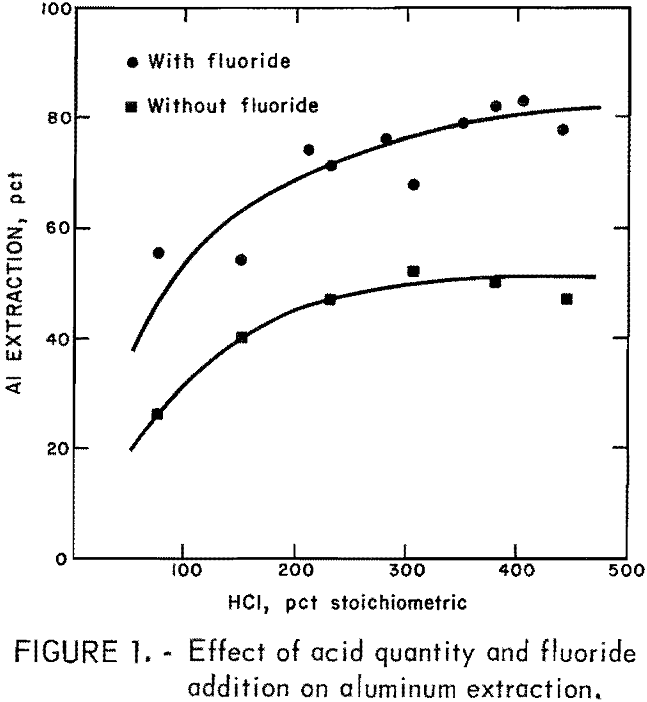
Anorthosite was leached with a constant quantity of HCl and variable amounts of H2SiF6. Fifty-gram samples were mixed with 300 ml of 20-pct HCl (210 pct stoichiometric to react with all alumina in the sample) and leached for 4 hr. Test results are summarized in figure 2.
Increasing the amount of fluoride increased aluminum extraction up to approximately 72 pct, but further fluoride addition did not. For single-stage leaching a F/Al ratio of 0.25 to 0.30 was required.
Aluminum extraction was studied as a function of retention time. Fifty-gram samples of anorthosite were leached with 300 ml of 20-pct HCl containing H2SiF6 at a F/Al ratio of 0.27. Results from leaching cycles of 1 to 20 hr are shown in figure 3. Since some aluminum extraction occurred during the 30-minute
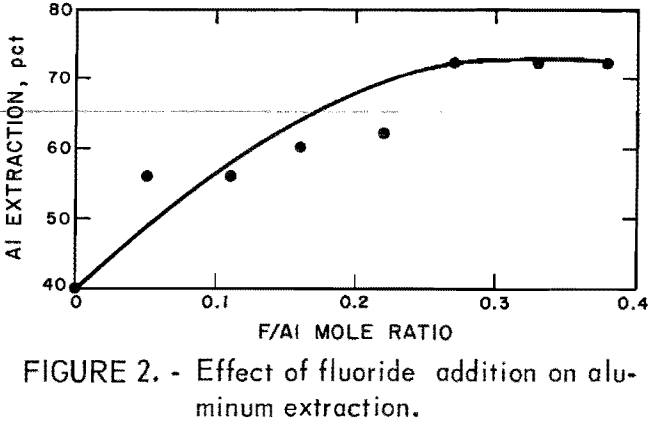
heat-up period, the curve does not intersect the origin. A steady increase in extraction occurred when retention time was increased. After 13 hr of leaching, 93 pct of the aluminum had been extracted; further leaching produced little more aluminum. Approximately 60 pct of the fluoride added to the acid solution remained in the pregnant liquor, while 40 pct precipitated and reported to the residue.
Cocurrent Leaching
Anorthosite was leached cocurrently by dividing the amount of leaching acid used in single-stage leaching into three fractions and applying them separately. A 50-gram sample was consecutively leached with three 100-ml portions of 20-pct HCl and added fluoride. The total of 300 ml of leaching solution contained 210 pct stoichiometric addition of HCl and a F/Al ratio of 0.27. Leaching time for each stage was 4 hr, or a total leaching time of 12 hr. Cumulative aluminum extraction reported in table 2 is 91 pct for the three stages. This was expected from figure 3 for single-stage leaching of 12 hr.
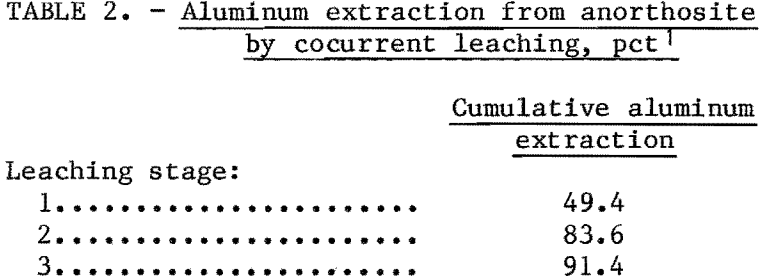
Countercurrent Leaching
Countercurrent systems are sometimes employed in the mineral industry in extracting and washing operations. Simulated countercurrent extraction of aluminum from anorthosite was studied by an extension of a multiple contact method outlined in the literature. The model in figure 4 schematically illustrates simulated three-stage countercurrent leaching by a series of batch tests. Fresh HCl leachant moves downward; anorthosite ore moves to the right. The two upper rows prepare leaching acid for use in the bottom row, where ore is contacted by acid of increasing strength. Since the spent liquors from the initial first and second rows are not utilized in the stages immediately below, they are discarded. Aluminum extraction was calculated from the head assay and the amount of aluminum remaining in the residue from the bottom row of leaches. Fluoride was added to the fresh leaching acid. H2SiF6, Na2SiF6, and CaF2 were used as sources of fluoride, and at equivalent amounts of fluoride addition no difference was noted in their effect. Retention time for each stage was 2 hr, or a total leach time of 6 hr.
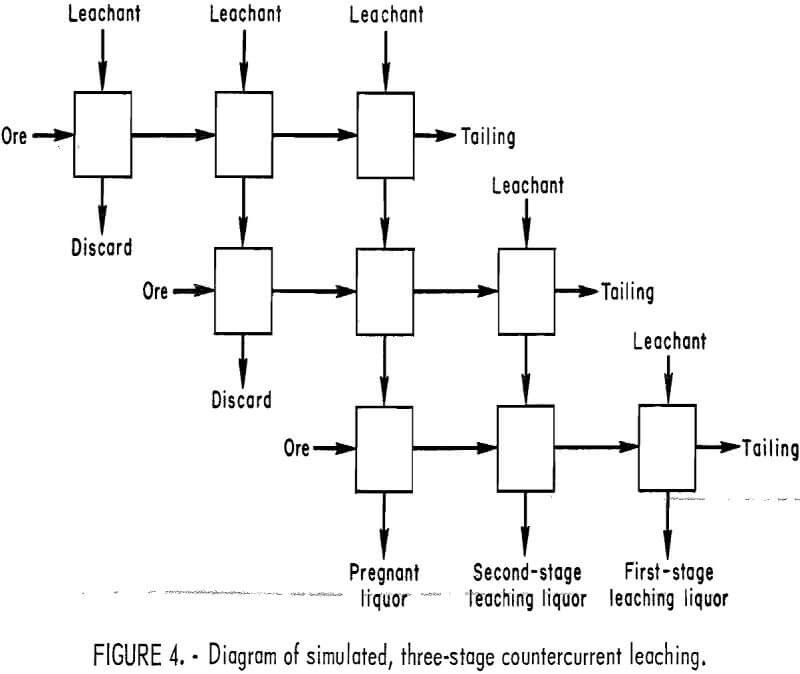
Table 3 summarizes the results of the simulated three-stage countercurrent tests using different amounts of acid and fluoride. Aluminum extraction was improved significantly compared to single-stage leaching using a corresponding amount of acid and fluoride and a 4-hr retention time. For example, 300 pct stoichiometric acid at a F/Al ratio of 0.27 was needed to achieve 75-pct aluminum extraction in a single stage. Test 8 in table 3 shows that 78-pct aluminum extraction was achieved with only 95 pct stoichiometric acid at a F/Al ratio of 0.07. More than 90-pct aluminum extraction can be maintained if either an excess of HCl or a high F/Al ratio is used. Tests 7, 8, and 9 show that if both acid and fluoride are decreased sufficiently, aluminum extraction decreases.
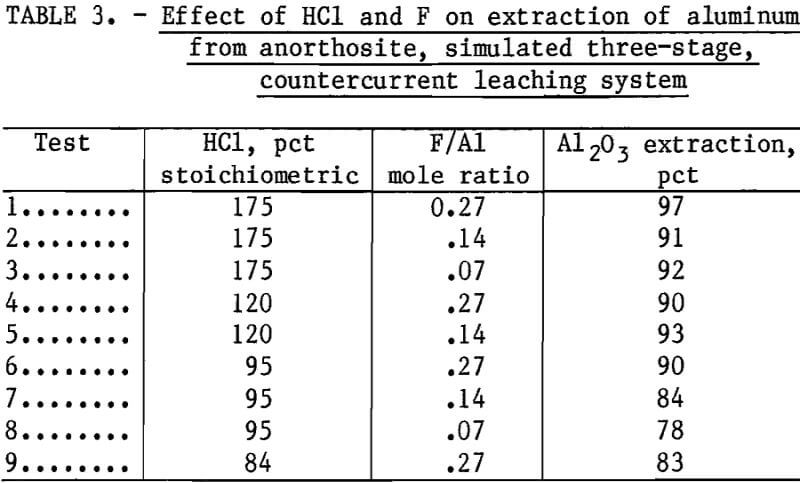
Utilizing conditions in which 90 pct of the aluminum was extracted, the other constituents were leached as follows:
CaO, 96 pct; Na2O, 74 pct; Fe2O3, 96 pct; K2O, 89 pct; MgO, 100 pct. A liquor having the approximate composition shown in table 4 was obtained. Since the liquor contained appreciable amounts of calcium, sodium, and iron chlorides, the liquor must be processed to separate the aluminum from these components. Tests have shown that most of the components can be separated by crystallizing AlCl3·6H2O by HCl gas injection into the liquor. AlCl3·6H2O and NaCl crystallized out, whereas the other components remained in the mother liquor. Sodium can be separated from the aluminum by calcining the chloride salts and water washing the calcine. Water-soluble NaCl is separated from the Al2O3, which is insoluble.
Summary and Conclusions
Excellent aluminum extractions were obtained from Wyoming anorthosite by countercurrent leaching with hydrochloric acid and fluoride ion. The leaching process eliminates the energy-intensive step of high-temperature calcination. Countercurrent leaching resulted in a more efficient use of acid and fluoride than single-stage leaching. Ninety percent of the aluminum values were extracted with 95 pct of the stoichiometric HCl requirement and a F/Al ratio of 0.27. If acid concentration is increased, the fluoride addition can be decreased. Three fluoride sources, CaF2, Na2SiF6, and H2SiF6, were studied and were equally effective.
