Table of Contents
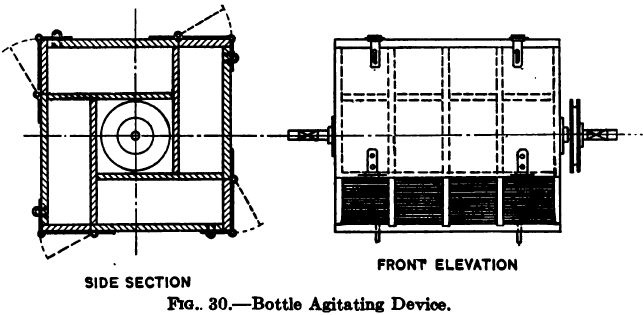
In the old days laboratory tests were usually made by mechanically shaking up in a bottle for a given time a charge of ore and cyanide solution. The most generally convenient device for this purpose is a wheel, to which are attached boxes, each capable of containing a standard acid bottle, and with means for securing the bottle firmly in place. The wheel is rotated at about 30 r.p.m. by a belt driven off a line of shafting. Objections have been raised to this device on the ground that agitation in a stoppered bottle does not allow of a proper aeration of the charge. Such objections, while plausible, are not borne out by facts, actual experience showing that the aeration obtained in this form of agitation is amply sufficient for almost any ore. It is of course necessary to leave an adequate air volume in the bottle, and for this reason the charge should not more than 1/3 fill it. A useful form of agitating wheel is shown in Fig. 30.
A form of bottle agitator devised by G. H. Clevenger is useful in some cases. It was designed to meet conditions where the material to be cyanided displays exceptional reducing tendencies and absorbs an abnormal quantity of oxygen, though the writer has found that in some cases it seems to give a less efficient aeration of the pulp than the usual agitation as already described.
It consists of two or more horizontal rollers 6 or 8 inches in diameter and of any desired length, placed parallel to one another with 2 or 3 inches clearance between their peripheries and belted to a line of shafting. The charged bottles are laid, unstoppered, horizontally on the space between two rollers, and thus rotated for any desired period.
When this device is used in warm dry climates the evaporation is so considerable during a two or three day agitation that calculations as to cyanide strength and consumption are rendered quite unreliable, and to correct this it is necessary to weigh the bottle at intervals (and especially at the end of treatment), and add sufficient water each time to compensate for the evaporation loss, the original weight of bottle and charge being of course known.
If the preliminary test seemed to indicate that excessively fine grinding was not necessary, it is probable that it will be more profitable to separate the pulp into sand and slime, percolating the former and agitating the latter.
As already stated, a good idea of the extraction to be expected by leaching may be obtained by agitating the sample in a bottle.
Actual leaching experiments on a laboratory scale, with charges of one or two kilos, may be made to confirm this fairly satisfactorily if proper precautions are taken. The usual method is to use a glass percolator or an inverted acid bottle whose bottom has been cut off, placing a filtering medium in the small end, and fitting a cork in the neck, through which passes a piece of glass tubing tipped with rubber tube and a screw clip. About 3 kilos of the ore, ground to No. 30 sieve are taken and the slime and — 200 sand washed out in water through a 200 sieve, or the slime alone may be removed by panning. (This water may be eventually used for making up the cyanide solution.) The — 200 material or slime is settled with lime water and decanted, and the +200 material or sand is drained as free from water as possible by continued tapping in a gold pan and pouring off the supernatant liquor. In this way sufficient moisture may be removed from the sand to permit of its being well mixed by rubbing with the hands. A sample for assay is taken of both sand and slime. Of the thickened slime one or more bottle charges are made up for an agitation test, and the sand, or an approximately known weight of it, is mixed with a suitable amount of fine lime, and charged into the percolator, each layer being firmly pressed down with the foot of a measuring cylinder or other tamping device.
A certain amount of cyanide solution is poured on each day and allowed to percolate slowly through, the daily proportion being regulated by the number of days treatment and ratio of solution decided on. Such ratio may be from ¾ to 1½ times the weight of ore taken for percolation.
If there is not too much fine sand in the pulp to interfere with percolation the separation into sand and slime may be made by merely washing in the gold-pan, though in this method most of the fine sand will remain in the portion to be percolated while in practice a large part of it would pass over into the agitation product.
In the case of readily oxidized ores it is sometimes more convenient to avoid handling wet products before treatment, and separation may be made by dry-screening the sample with a 200 or 150 sieve.
One of the principal difficulties in such percolation tests lies in the fact that the charge is so much shallower than a working charge that there is not sufficient head to overcome the capillarity of the interstices, so that, even with sand coarse enough to percolate very rapidly, the level of solution will not fall much below the level of the sand. The result of this is that the charge is not aerated (as it is in practice), by the air following the solution down into the interstices, between each wash. This may be overcome by applying a vacuum under the filtering medium after the solution has ceased to percolate by gravity. In this way the residual solution is drawn off and the air follows it down. The charge should then stand for several hours before the next wash is applied. This procedure is more important than it may seem, since a difference in extraction of 20 per cent, to 30 per cent, has been in some instances observed according to whether the vacuum was applied or not.
Another difficulty with small-scale leaching tests lies in the evaporation of solution, which is excessive, and out of all proportion to the size of the charge, rendering determination of the cyanide consumption quite unreliable unless careful measurement be made of the solution coming off, and the deficit made good by addition of water. Even so, the figure obtained is to be received with caution, as it is usually considerably in excess of working- scale results.
The most reliable way to conduct a leaching experiment is to use a vertical piece of iron pipe the depth of the tank proposed to be used in the plant, say, 6 ft. high, fitted at the bottom with a sleeve and plug, on top of which is laid a filtering medium. The plug is bored in the centre to admit a small iron cock. The diameter of the pipe should be at least 6 inches, because if too small the sand will not pack to a normal density, and will also be difficult to discharge when finished. Even with a 6-in. pipe it is often advisable, if charging with damp sand, to pack each layer gently down with a broom handle so as to approximate to the density found in a large working tank. Such a leaching test will need from 50 to 100 lb. of sand for each charge, and of course will necessitate provision for grinding and classifying several hundred pounds of ore at a time.
Concentration Tests
If the agitation tests on the sample of ore ground to pass 200 sieve are not satisfactory, it will be necessary to try the effect of concentration. For this purpose take about 500 grams of ore, crushed to No. 30 sieve, and carefully pan twice over. The tailing may then be ground in water or cyanide solution as already described and screened wet through a No. 80 or 100 sieve, and again panned. To assist the operation of panning the —100 product, a large proportion of which will be slime, the fine part may be floated off and run over a little canvas table, leaving the granular part only to be panned. In this case, however, the product of the canvas table will need to be cleaned in the pan, but this is simpler and less tedious than panning the whole. The tailing, after being sampled for assay, may be cyanided as it is, or first ground to pass a No. 200 sieve.
Lead Salts
When experimenting with silver ores some charges should always be made up with the addition of varying amounts of lead acetate or litharge as the presence of lead will often be found a considerable aid to extraction of the silver, particularly when there is no zinc present in the solution.
If litharge be used for this purpose it should be ground fine, say to pass 200 mesh.
In the case of many ores a critical point in the addition of lead compounds is reached which yields the maximum increase in extraction and beyond which the improvement due to its use declines until a point is reached when the extraction is no better and sometimes even worse than without the addition of lead so the determination of the right amount of lead compound to be used is a matter of great importance.
Test for Repeated Use of the Same Solution
In dealing with any unknown ore there is an important experiment which should never be neglected. It sometimes happens, especially with ores containing arsenic and antimony, that a test made with new solution will give a high extraction, while a similar test made up with solution which has been used over several times after precipitation with zinc will give a much lower one. In the case of such ores the presence of lead is often as inimical to a good extraction as the presence of zinc.
To make this test it is necessary to start the series with several bottle charges so as to furnish sufficient solution with which to carry through the experiment. Each time the solution is used there is a loss due to withdrawals for titration and other causes. The first of the series may be made with 4 bottles, charged in the usual way, the solution after treatment and precipitation with zinc dust being made up to cyanide strength and used to treat the next series of perhaps 3 bottle charges of the original size or 4 smaller charges, and so on until the same solution has been used over 5 or 6 times, by which time the original solution will probably have shrunk to only sufficient for one bottle charge. If by the 6th round no deterioration in extraction has been noted it is safe to assume that the activity of the solution will not be impaired by repeated use on a working scale, under the conditions of zinc precipitation. For this experiment the use of a laboratory vacuum filter is almost a necessity.
The whole amount of wash, however, necessary for displacing the dissolved metals preparatory to the assay of the ore residue should not be added to the stock solution as that would dilute it out of all proportion to milling conditions. Assuming that the filter cake when firmly set contains about 25% moisture, and working with a 250-gm. charge of ore 83 cc of water would be first put on and allowed to leach through, to be at once removed from the receiver and added to the stock solution. After this ample water washes are given to ensure the complete removal of soluble gold and silver from the cake.
Use of Working Plant Solution
When making experiments on an unknown ore using barren solution from a working plant where zinc precipitation is used a serious error in the calculation of cyanide consumption is likely to occur if the figures are based on the titrations for free cyanide, because when such solution comes in contact with the lime added in making up a bottle charge some of the zinc-potassium cyanide will be regenerated and recorded as free cyanide, and thus the consumption will appear much less than the actual figure should be. This may be avoided by making a complete set of titrations for total cyanide when making the usual ones for free cyanide, and then basing the consumption calculation on the readings for total cyanide.
When the ore, however, itself contains zinc soluble in cyanide solution it is practically impossible to estimate the cyanide consumption correctly when using barren plant solution.
Larger Agitation Charges
Where there are conveniences for grinding and classifying several hundred pounds of ore at a time it is often of advantage to check the results of bottle tests by charges of 50 to 100 lb. For this purpose it is easy to fit up some small tubs of wood or sheet iron and fix mechanical stirring devices in them. Such mechanical agitation is sufficient to give the maximum extraction in many cases, but with ores that have a reducing tendency it is often necessary to supplement it with an air lift, to facilitate aeration of the charge. If there is a workshop available it is not difficult to have some small Pachuca tanks made out of sheet iron, and these are often very useful for experimental work. When using such tanks in dry climates it is needful to be on the watch for considerable evaporation, resulting in a decreased ratio of dilution, and consequent concentration of precious metal and cyanide content in the solution.
It is also necessary, as already pointed out, to see that the chances for an accumulation of dead pulp in the cone are reduced to a minimum as this is a fertile cause of salting the residue sample.
