When a mineral particle is fractured, bonds between the atoms are broken. The unsatisfied forces that appear at the newly formed surface are considered to be responsible for the adsorption of ions at the mineral surface. A knowledge of the mechanism and extent of ion sorption from solution onto a mineral surface is of interest in the development of the theory of flotation.
Hydrogen and hydroxyl ions are always present in an aqueous solution. By controlling the pH, the concentration of these two ions was kept constant. The variation in the amount of sodium adsorbed with variation in sodium concentration was then determined under conditions standardized in regard to hydrogen ion. The effect of concentration of hydrogen ions and of other cations was also measured. A few experiments were made to get a preliminary idea on the effect of anions.
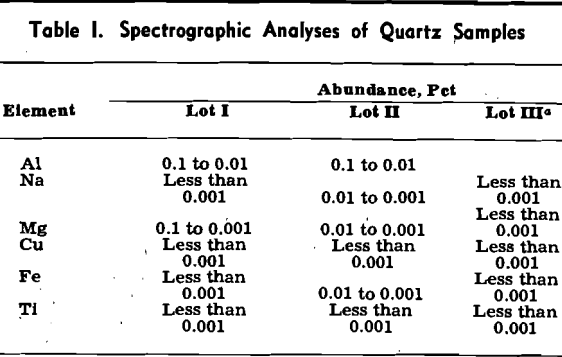
The quartz was prepared as in previous work in the Robert H. Richards Mineral Engineering Laboratory’ except for the refinement of using de-ionized distilled water for the final washing of the sized quartz, prior to drying. To minimize the laborious preparation of quartz, experiments were made to determine whether the sodium-covered quartz could be washed free of sodium and re-used. The experiments were successful as indicated by lack of Na22 activity on the repurified material and by its characteristic sodium adsorption.
Method of Beta Counting for Adsorbed Sodium: Na22, the radioisotope of sodium, possesses convenient properties. It has a half-life of 3 years, thus requiring no allowance for decay during an experiment. On decay it emits a 0.575 mev β+ radiation and a 1.30 mev γ radiation. The decay scheme is illustrated in the following equation:
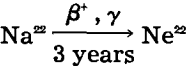
The β radiation is sufficiently strong to penetrate an end-window type of Geiger-Mueller counting tube. This, in turn, makes it possible to use external counting, a great advantage in technique. Furthermore, it permits the assaying of solids arranged in infinite thickness, while assaying evaporated liquors on standardized planchets.
Procedure for Adsorption Tests: The method consisted of agitating 12 g of quartz with 60 ml of solution of known sodium concentration for enough time to establish equilibrium between the solution and the quartz surface. The quartz was separated as completely as possible from the solution by filtering and centrifuging. The activity on the quartz and in the equilibrium solution was measured and the partition of the sodium was calculated from the resulting data.
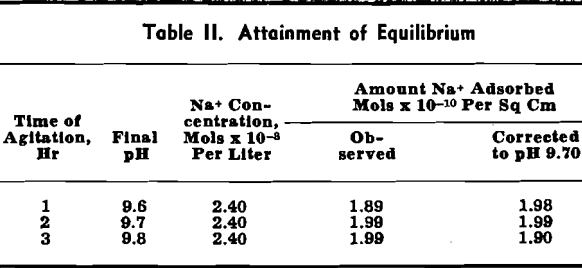
Attainment of Equilibrium: Three adsorption tests were conducted in which the only variable was the time of agitation. The tests were run for 1, 2, and 3 hr. The results obtained are summarized in Table II. This table shows that the amount adsorbed (col. 4) does not differ from test to test significantly more than the pH (col. 2). As is shown below, the amount abstracted varies as the pH. If the data of col. 4 are corrected to the same pH (9.70) as explained further, these data support the conclusion that agitation for 1 hr is sufficient for equilibrium.
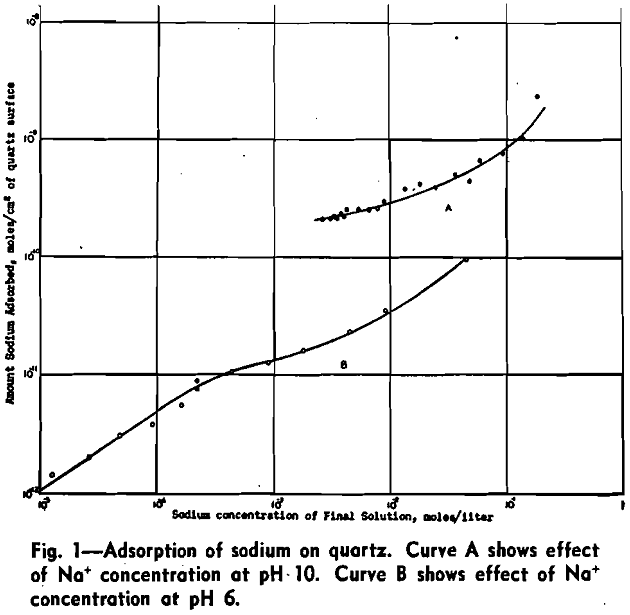
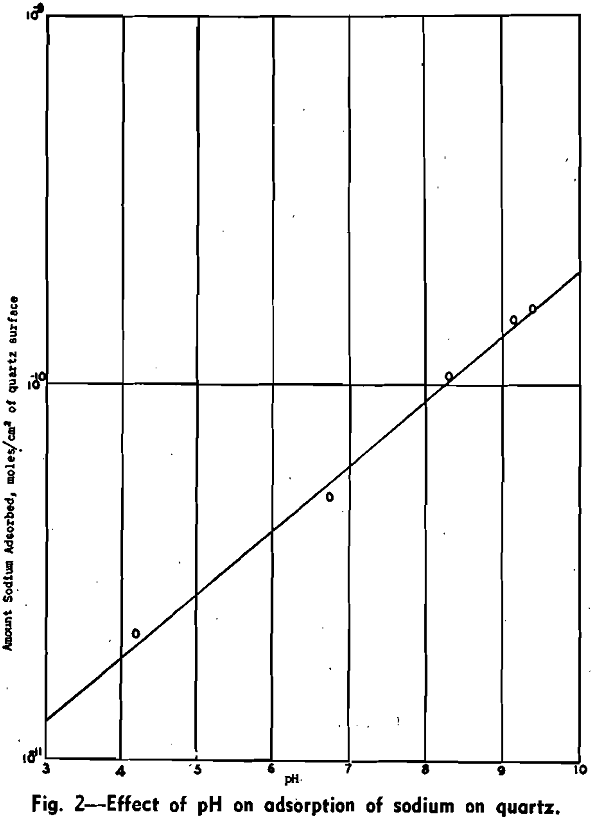
Effect of pH: One series of tests was conducted in which the sodium concentration was kept constant while the pH was varied with dilute nitric acid. The results obtained are given in Fig. 2. The amount of sodium adsorbed decreases with increase of hydrogen-ion concentration.
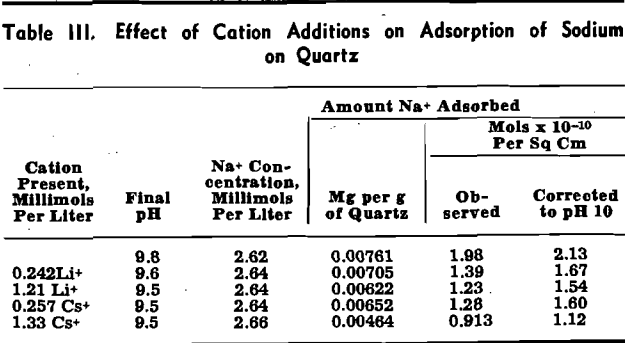
Effect of Variation of the Predominant Anion Present: Six tests were conducted in which standard sodium solutions other than sodium nitrate were added.
The present-day concept of adsorption at a solid-liquid interface visualizes the existence of a so-called double layer of ions at the interface.

This double layer is thought to be made up of an inner part attached to the surface and an outer diffuse part. For a quartz-solution interface the ζ potential is negative (—44 mv according to Freundlich).
When a quartz particle is placed in pure water, hydroxyl and hydrogen ions are adsorbed onto the surface and into the diffuse layer. A dynamic equilibrium is established between the ions in the solution and those on the surface. The presence of other ions would alter this equilibrium, the new equilibrium depending upon the nature and concentration of the ions in the solution.
