Table of Contents
The Nature of Activated Carbon
“Activated carbon” or “activated charcoal” is a generic term applied to amorphous carbon materials that have adsorptive capability. These materials are made from different sources and are available as powder, pellets, or grains. Their common characteristic is a very large surface area in the range of 600 to 1200 m²/g that arises from an enormous porosity. The activated carbon most commonly used in the CIP process is the gas adsorbent coconut carbon, which has slit¬like pores in the micropore range.
During processing, activated carbon irreversibly adsorbs oxygen in the form of H2O, O2, or CO2. Because of the disordered lattice of activated carbon and the presence of numerous broken C-C bonds, oxygen can combine with the carbon in may different ways, thus, producing a broad variety of functional groups. Of these, phenolic and carboxylic groups have been suggested. Also believed to be present are quinone-type carbonyl groups, carboxylic acid anhydride, cyclic peroxide, normal lactones, and fluores- cein-cype lactones.
Experimental Materials and Methods
The activated carbon used in these experiments was an 8 x 10 mesh coconut carbon, NACAR G210R, supplied by North American Carbon, Inc., of Columbus, Ohio, USA. It was cleaned by soaking samples of around 200 grams in 1 liter of molar hvdrochloric acid solutions for 24 hours. Each sample was then repeatedly rinsed with triply distilled water until no chloride could be detected in the supernatant. Rinsing was continued by soaking the samples in triply distilled water for 15 days, with the water being changed daily. The pH and conductivity of the supernatant were measured just before the water change. Rinsing was assumed complete when the pH and conductivity leveled off with time. Finally, the samples were dried under vacuum at room temperature and stored under nitrogen.
Silver cyanide solutions were prepared by dissolving AgCN in cyanide solutions so that the final Ag concentration was 0.1 molar at a CN:Ag ratio of 10 (except when otherwise specified). Silver cyanide solutions of various strengths were prepared by dilution from this stock solution. Copper and mercury cyanide solutions were prepared in the same manner from solid CuCN and Hg(CN)2, respectively. Stock solutions for zinc and cadmium cyanide solutions were prepared by dissolving their oxides (ZnO and CdO) in cyanide solutions. As before, the total metal concentration in each solution was 0.1 molar and the CN:metal ratio was 10.
All chemicals used in this work were of reagent grade except AgCN and CuCN, which were of purified grade and technical grade, respectively.
Characterization of Activated Carbon
The BET surface area of the activated carbon with nitrogen gas as adsorbate was 532 m²/g. With carbon dioxide as the adsorbate, 1220 m²/g was obtained using the Dubinin-Polanyl Equation. If the difference between the two areas can be taken as the area in the micropores of the activated carbon, then these results indicate that about 60 percent of the total area is found in the micropores.
Adsorption Isotherm
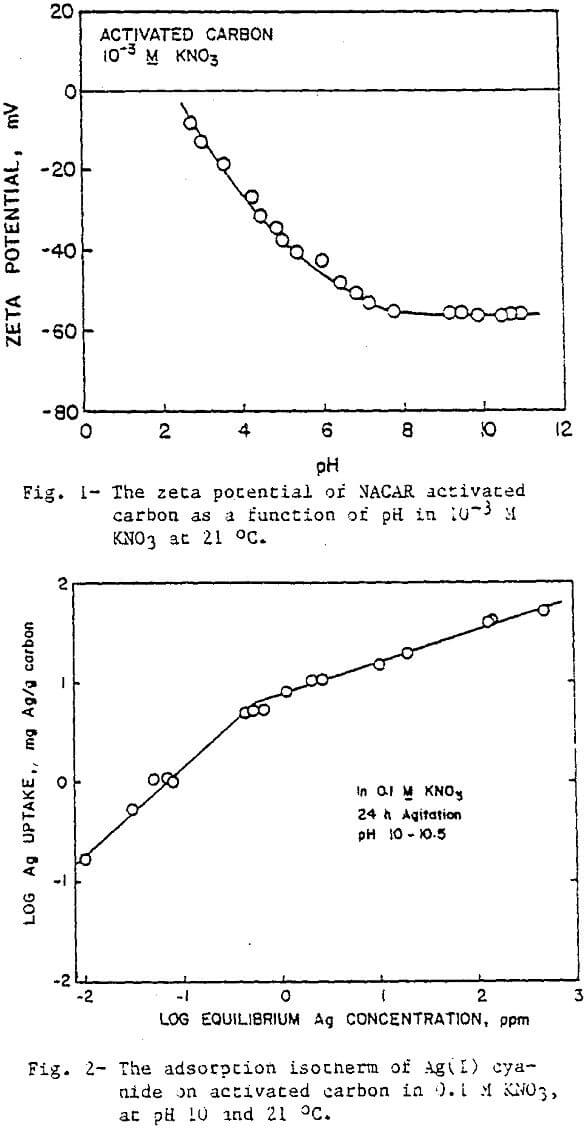
The adsorption of Ag(CN)2 on activated carbon can be represented by the Freundlich Isotherm as indicated by the straight lines in Figure 2, which plots the amount adsorbed vs. the equilibrium concentration on logarithmic scales. Monolayer coverage is attained when 0.5 meq or Ag is adsorbed per gram of carbon. This translates to an area of about 4 nm² occupied by each adsorbed molecule.
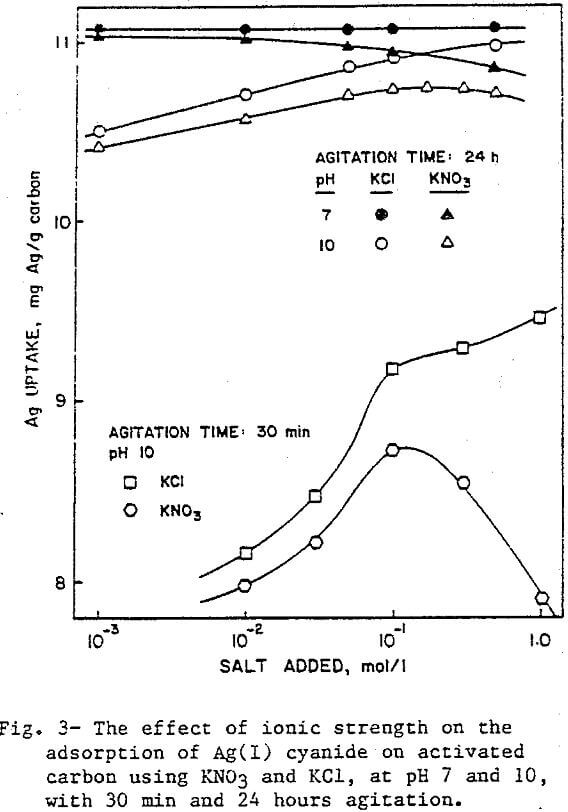
Effect of Ionic Strength
In general, the amount of silver adsorbed on activated carbon increased with increase in ionic strength. Figure 3 illustrates the effect of the ionic strength on adsorption using KNO3 and KCl at pH 7 and 10. At pH 7, the maximum recovery is attained at the lowest ionic strength with either salt. As the ionic strength was increased with KCl, the Ag uptake remained unchanged, but with KNO3 a decrease in adsorption was observed. At pH 10, both lines are shifted to lower values because of the slight increase in the negative charge of the activated carbon. This time KNO3 improves the recovery up to a concentration of about 0.2 M. On the other hand, the line for KCl shows increasing recovery, levelling off only as the recovery approaches the maximum, and never decreasing.
The difference in the affects of KCl and KNO3 on the adsorption is probably a result of the differences in their size and bonding behavior. The aqueous ionic radii of Cl- and NO3- are 0.183 and 0.206 nm, respectively. The larger radius of NO3 makes it more effective in blocking the pores of activated carbon than Cl-.
Effect of pH
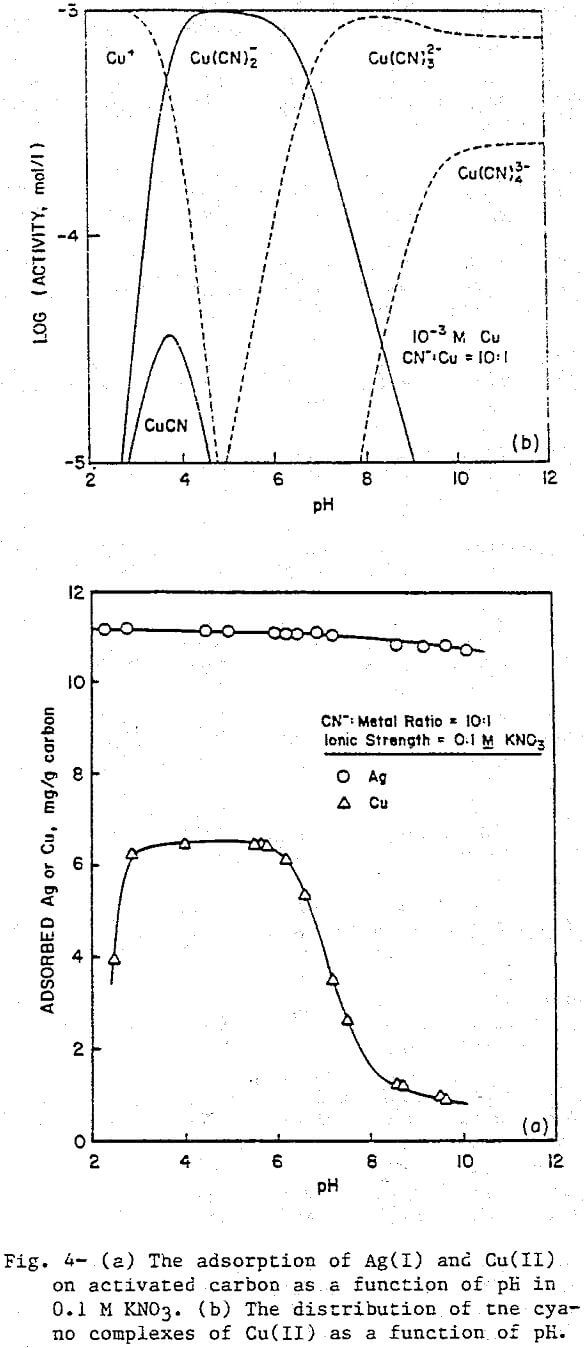
The uptake of Ag(I) cyano complexes by activated carbon is shown in Figure 4a. Adsorption is essentially complete throughout the pH range of the experiment. In the same range, the predominant form of Ag(I) is Ag(CN)2, evidence that the adsorbing species is the Ag(CN)2 complex.
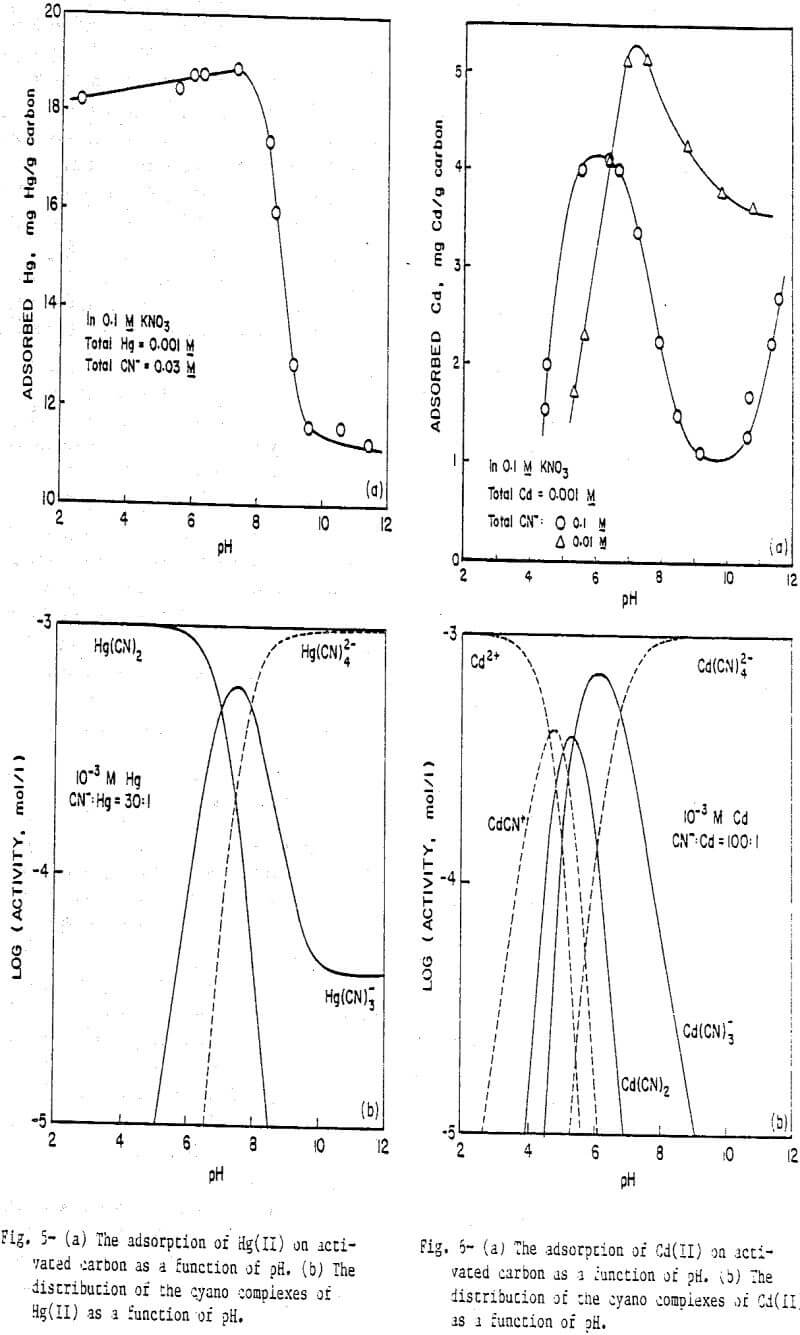
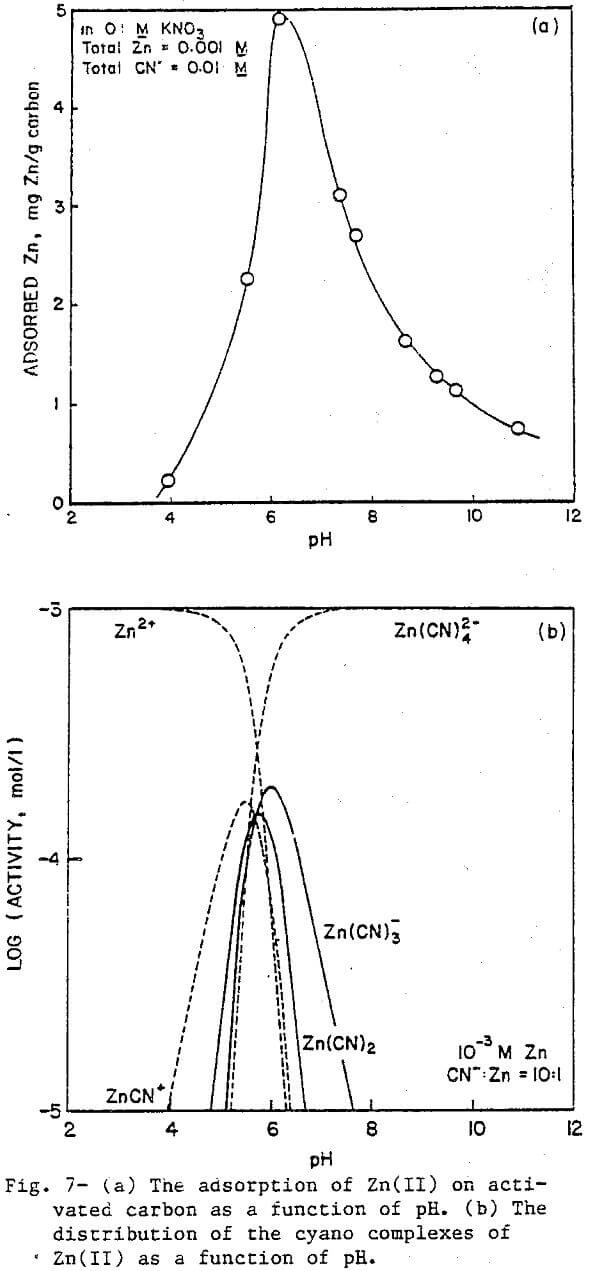
The cyano complexes of Group II-B metals introduce a slight variation because, for the same coordination number, each complex will have one less negative charge compared to the cyano complexes of Group I-B metals. Figure 5a presents the results for Hg(II) cyanide adsorption on activated carbon at a CN:Hg ratio of 30, with the corresponding distribution diagram being given in Figure 5b. Again, the agreement between the adsorption data and the predominance lines for Hg(CN)2 and Hg(CN)3 is good. The tetracyano complex which has a —2 charge does not adsorb while the neutral Hg(CN)2 and the negatively charged Hg(CN)3 do adsorb. No sharp drop in adsorption is seen at low pH values because no positively charged species exists in significant amounts under these conditions. The very low concentrations of Hg²+ and HgCN+ arise from the fact that the first two stability constants for the Hg(II)-cyanide system are so large (log K1 = 18.0, log K2 = 16.70) that the overall reaction to form Hg(CN)2 essentially goes to completion.