Table of Contents
Sulfonate Collector
Data on sulfonate adsorption by silicate minerals are very scarce in the literatures. The experiments were divided into two groups. The first group included the study of the flotation response of plagioclases as a function of pH and sulfonate concentration. This group also included the study of the effect of Ca(II) and Al (III) cations as activators for albite flotation as a function of pH and cationic concentration. The second group of experiments was designed to include the determination of the amount of sulfonate ion consumption from, as well as the rate of appearance of cationic species in the water phase.
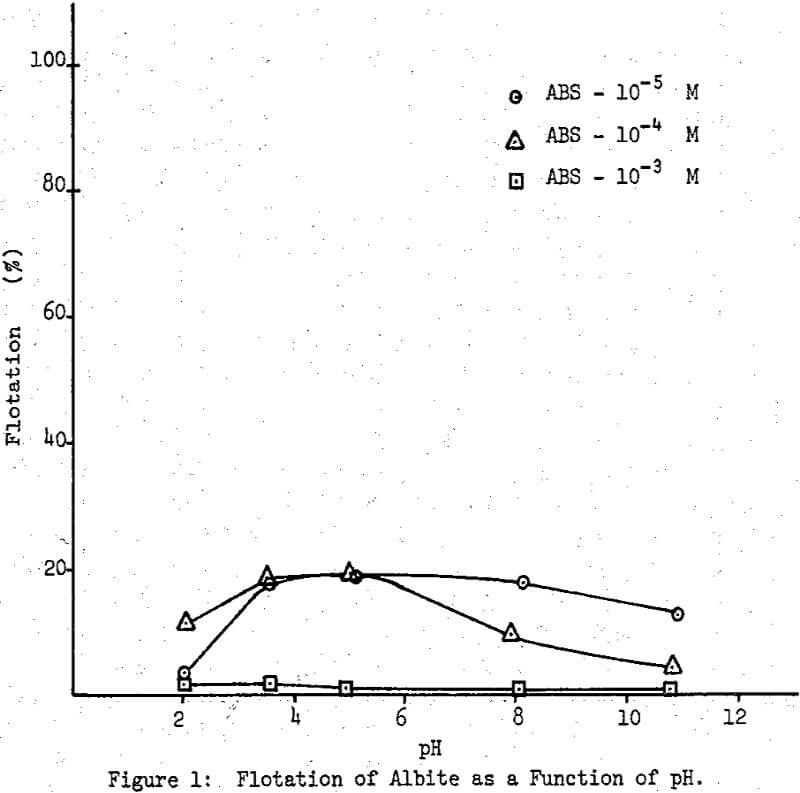
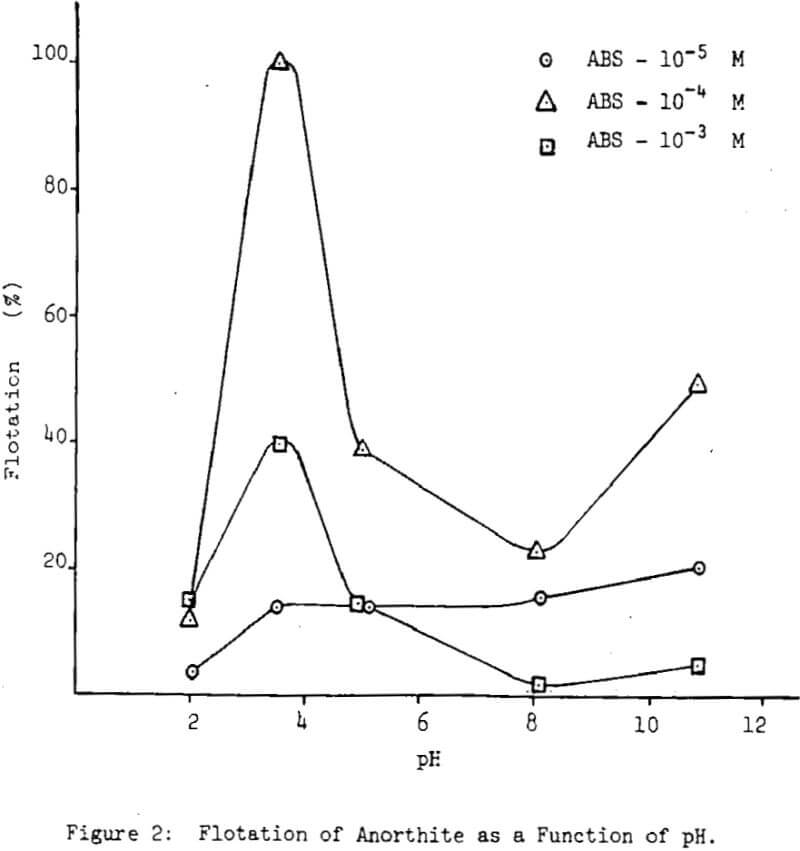
The series of flotation experiments included three minerals, albite, anorthite and labradorite. Three sulfonate concentrations, 10 -5M 10 -4M and 10-³M were used over a wide pH range. All the results are reported as percent flotation.
The test conditions for albite flotation were found to be a pH 4.0 with a sulfonate concentration of 10 -4M, where about 20 percent of the material floated. Below and above this pH value flotation recovery decreases, more drastically at low pH values than at high ones. In the case of anorthite, complete flotation was obtained at pH 3.5 and a sulfonate concentration of 10 -4M. Below this pH, the recovery drops sharply to about 10 percent, while above the same pH, there is a gradual decrease to about 40 percent recovery at pH 5.0 and as low as 23 percent at pH 8.0; higher pH values for the solution resulted in an increase in flotation recovery to about 50 percent at pH 10.8.
Three pH values, 2.0, 3.5 and 8.0 and three sulfonate concentrations, 10 -5M, 10- 4M and 10-³M were chosen for the study. The pH values corresponded to those where the flotation response showed a maximum or a minimum. All the results are reported in meq/g for the adsorption density units and hours were used as time units.
Discussion of Results
Although the plagioclase-sulfonate interface proved to he one of the most difficult systems under study, the flotation results clearly indicate the importance of the following four factors:
- The composition of the plagioclase and the extent of its solubility in in aqueous media.
- The relative concentrations of Ca(II) and Al(III) cations in the solution phase.
- The concentration of sulfonate ions in solution.
- The pH of the system.
The fact that plagioclases floated well at the pH values where hydrolysis of Ca(II) and Al(III) begin to occur, seems to support the explanation given by Fuerstenau and Bhappu for the activation of quartz by these cations. This idea was further substantiated by the activating effect that Ca(II) and Al(III) have on albite at the pH values mentioned.
The activating effect of Ca(II) on albite at pH 5.0 was surprising owing to the fact that at this pH value the predominant Ca(II) species in solution should be Ca+², and if a solid metal-sulfonate precipitates it should be of the form Ca(RSO3)2 which is not likely to possess collector properties.
A sulfonate concentration of 10 -5M would be too low to produce the necessary amount of cation-sulfonate basic salt to act as collector species. Under such conditions, extremely high cation concentrations of either Ca(II) or Al(III) would be needed in solution to meet the sulfonate demand, and, as shown by Escalera no plagioclase is that soluble so as to meet this demand unless the pH value is very low, but under conditions of low pH no hydroxy-sulfonate compound can be formed; thus, we again lack cation-sulfonate species with collecting properties.
It is also evident that collector attachment on plagioclase surfaces is possible only when the solubility product of either aluminum-sulfonate or calcium-sulfonate has been exceeded, and this could occur only when enough Al(III) or Ca(II) have been released from the solid phase to produce such excess. Therefore, a plagioclase low in these cations could be expected to respond poorly to flotation by a sulfonate collector. In this respect, the flotation response of plagioclases should increase in the order albite → labradorite → anorthite and the experimental results show precisely this order of flotation response.