Activated bleaching clays, so called because of their ability to remove color bodies from bleach fats and oils, are produced from clays of bentonitic origin. Activation is achieved by subjecting these clays to physical or chemical alteration to increase selective sorption capacities.
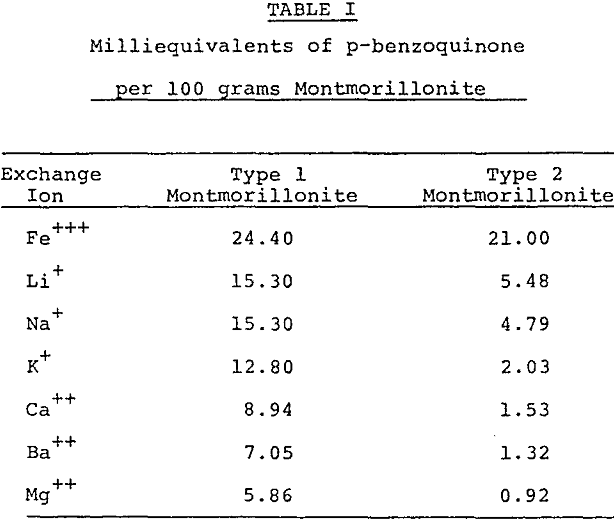
Bentonitic clays which are suitable for production of activated bleaching clays contain the essential montmorillonite clay mineral as well as substantial inert impurities. Activation of montmorillonite by acid leaching involves partial dissolution of the octahedral layer. The activity level attainable depends upon the concentration of the montmorillonite. A high activity clay cannot be obtained by acid activation of bentonite containing a low percentage of montmorillonite.
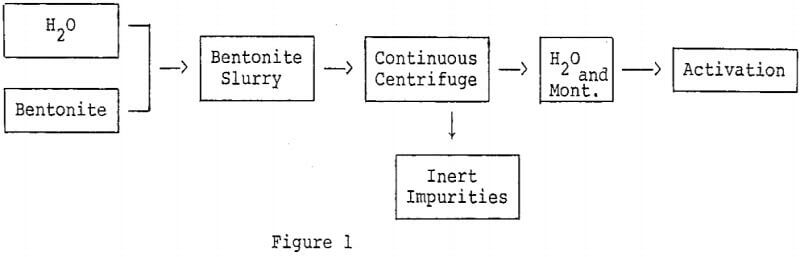
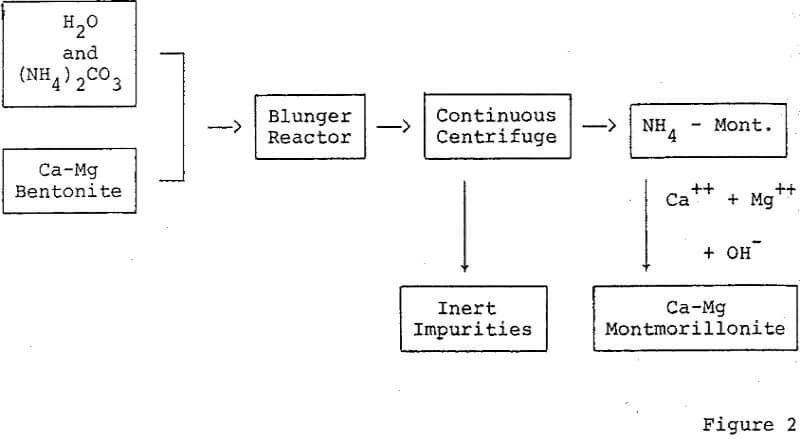
Removal of inert impurities from bentonite to effect concentration of montmorillonite is a method for improving the activity of bleaching clays. A simple technique which can be employed to concentrate certain montmorillonites is dispersion and centrifugation.
Further purification techniques may be applied to the acid activated montmorillonite. For Example, ion exchange resins may be used to remove interfering impurities remaining after the acid dissolution and rinsing procedure. The refined activated montmorillonite may be treated additionally by ion exchange to add important reaction promoting cations, for example, the Fe+++ ion as an oxidation accelerator.
Decolorization of fats and oils by activated bleaching clays will continue to be important. The more concentrated forms of activated clays should gain greater acceptance. These types of clays, although costlier to produce, have the advantage of greater decolorizing capacity, therefore requiring lower dosages. By virtue of using less clay, less oil is retained, which improves the yield of finished oil and requires less filter capacity.
Bentonites sorb or react with certain organic molecules to form organo-montmorillomte complexes. Primary, secondary, and tertiary amine salts, as well as quaternary ammonium salts form organo-montmorillonite complexes through cation exchange. Proteins, at or below the isoelectric pH, are rapidly complexed by montmorillonite through cation exchange involving the ionized amino group. These organo-montmorillonite complexes generally form flocced precipitates in water and are easily removed from suspension by filtration, centrifugation, or settling.
X-ray diffraction analyses have shown that organic anions do not enter between montmorillonite platelets, but are sorbed on the edges of the clay particles. The mechanism of organic anions bonding to the edges of montmorillonite has been considered as an electrostatic bonding of the anion to positively charged sites on the clay edges.