Table of Contents
In past years, water drainage control in coal mines has been primarily directed on the basis of water quantity rather than quality. Due to the recent intense interest in total ecology and environment, emphasis has shifted to the point where the water exiting from a mining operation may be of better quality than when it enters. In most operations this has meant the use of treatment methods (neutralization) while the technology for the prevention of acid drainage is being developed or refined. To date, underground abatement of acid formation has generally been less expensive but also less effective than surface treatment of the mine water.
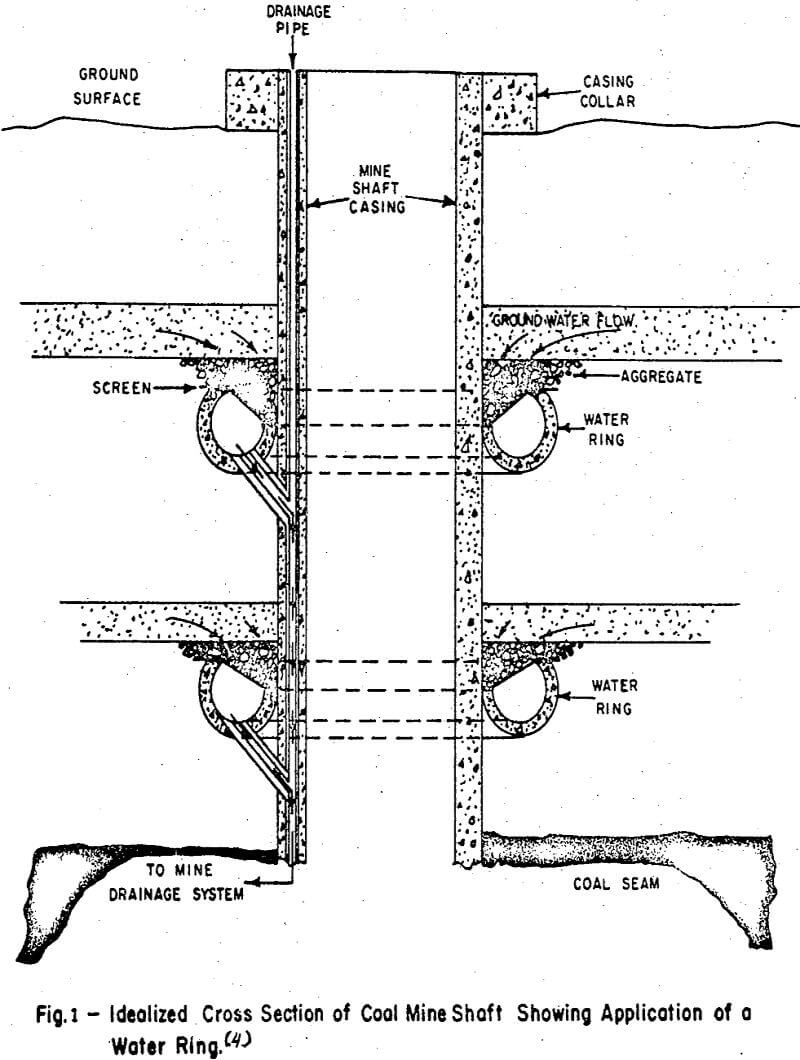
Restriction of Water Entry
During the construction and development of a coal mine, water may enter in several ways:
- From water bearing strata in contact with the coal seam;
- In shallow mines, from the surface;
- Through faults and fractures in coal seams and adjacent strata; and
- From contact with previously abandoned workings.
Although many of the sedimentary beds associated with coal deposits are relatively impermeable to water flow, sandstone can be very porous and an excellent aquifer capable of transmitting many thousands of gallons of water.
Treatment by Neutralization
Conventional surface treatment of acid mine water involves lime neutralization followed by settling of the precipitates formed. Generally, the lime Ca(OH)2 is slurried using either acid water or treated water. The lime slurry is added to the acid mine water in sufficient quantity to raise the pH above seven. The now neutralized water will be aerated to oxidize the ferrous iron to ferric iron and pumped into a settling basin.
Ferrous iron (iron in the +2 state) is soluble in water and must be converted to ferric iron (iron in the +3 state). Ferric iron is insoluble at higher pH values and will precipitate. Ferrous iron is difficult to oxidize at low pH values so lime is added to the acid mine drainage before aeration. It is necessary to add an excess of lime before aeration since oxidization of the ferrous iron is accompanied by the release of a hydrogen ion.
After neutralization and aeration are complete, the precipitate (sludge) and the water are separated by gravity settling. This may be done in either a clarifier or an earthen impoundment (lagoon). A clarifier is generally a circular concrete pond on the order of 100 feet in diameter and 10 feet deep. The exact size of the clarifier depends on the volume of acid mine water being treated and the settling rate of the sludge. In most cases the water is collected around the circumference of the clarifier and the sludge is mechanically raked to the center of the clarifier where it is pumped away for storage in an impoundment. Clarifiers are not always circular, some being rectangular in shape.
The limestone process for treating coal mine water involves essentially the same unit operations as are used in lime neutralization. The chief advantages of limestone treatment are that limestone is cheaper per ton and produces a denser sludge than does lime, thus reducing the sludge disposal problem.
Treatment by Reverse Osmosis
Neutralization systems for treating acid mine drainage have one common problem, they do not produce a water suitable for most domestic or industrial use. Neutralized acid mine water contains high dissolved solids, hardness and sulfate. One process which has been found to be highly effective in the removal of nearly all the dissolved solids in acid mine drainage is reverse osmosis.
Osmosis is flow from a dilute solution through a semi-permeable membrane to a more concentrated solution. In reverse osmosis (R.O.), a pressure is applied to the concentrated solution reversing the flow. The semi-permeable membrane can be selected so that only water will pass through leaving any dissolved solids behind.
The tubular configuration is merely a porous support tube lined with a membrane. The feed water enters one end of the tube and the product passes through the membrane and the porous tube. The product is collected by a plastic shroud. Generally, several tubes are in each plastic shroud.
Treatment by Ion Exchange
Another process for producing domestic or industrial water from acid mine drainage is ion exchange. Ion exchange is a process in which ions held by electrostatic forces to the surface of a solid are exchanged for ions of a different species in solution. An ion exchange material must contain an ion which can be exchanged for an ion in the solution being treated. These exchanged ions are later released by changing the chemical characteristics of the surrounding solution. Generally, an ion exchange process uses both a cation (positive ion) exchanger and an anion (negative ion) exchanger in series to affect a complete removal of dissolved solids from the solution being treated. It is possible to produce a water equivalent in purity to distilled water by ion exchange.

