Drainage of acid mine water into surface streams of coal mining areas is one of the most serious problems of stream pollution, since there is no known method that completely prevents its forming and no economically feasible treatment after it has formed. The mine acid problem differs from other pollution hazards because acid production does not end with cessation of mining but actually becomes more evident when pumping is stopped.
The effective acid is the result of an original oxidation of sulfuritic materials associated with the coal measures, usually FeS2 or pyrite. This reaction produces ferrous sulfate and sulfuric acid. Subsequent reaction of the sulfuric acid with the rock, shales, and limestones also associated with the coal measures converts it to aluminum, calcium, and magnesium sulfates. Analyses of hundreds of samples of acid mine discharge show that the amount of sulfate present is chemically equivalent to the sum of the metallic elements. It is usually considered, therefore, that there is no free acid.
One analysis, which can be duplicated for any sample, is important in determining not only the immediate effect of acid water entering the stream but also the ultimate effect. Total acidity is determined by titration in hot solution to a neutral end point, or pH 7.0, or for routine analysis and control, to phenolphthalein indicator.
In recent months the press has published reports that formation of acid can be stopped by use of inhibitors. This proposal is not new, but no such inhibitor has come to the attention of the writer or his colleagues. Moreover, application at the acid-producing spots in abandoned mines, the worst offenders, presents almost insurmountable difficulties, as such mines, being without maintenance or ventilation, are inaccessible because of roof-falls or gas.
There are three possible methods:
- introduction into the water entering the mine,
- application on the surface of the overburden with the possibility that the inhibitor will be carried into the mine by water seeping through the strata, and
- introduction as a gas that would permeate the mine by convection.
A small abandoned mine that had been under observation for two years was selected for the purpose. During the period of observation, flow and acidity were measured at monthly intervals. This mine had a maximum cover of about 200 ft. Very few falls were apparent in the accessible areas, accessibility being determined by the oxygen content of the atmosphere. Maximum human penetration was about 700 ft. According to underground maps the entire mine was open as far down as a borehole about 1700 ft from the entry. Oxygen content of the mine atmosphere at the borehole varied from 2 or 3 pct to 21 pct, indicating that the borehole opened into the mine and that the atmosphere was as variable as at other accessible points.
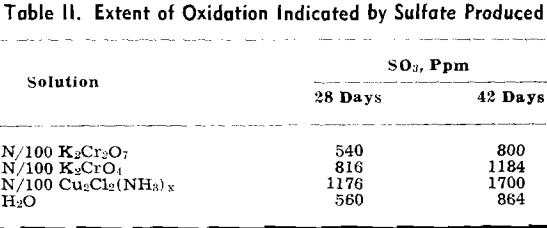
The volume of ammonia gas introduced into the mine was such that it should have been apparent at the entry had there been no obstruction between the borehole and the entry. Several efforts were made to reach the foot of the borehole from the entry, but a point was always reached where the atmosphere became so deficient in oxygen that safety lamps were extinguished. It is possible that an amount of acid equivalent to the amount of ammonia introduced had been neutralized in some area of the mine, but that area was separated from the accessible area by falls and thus there was no permeation of the ammonia or the water. The sulfate produced was used as a criterion of the extent of the oxidation.
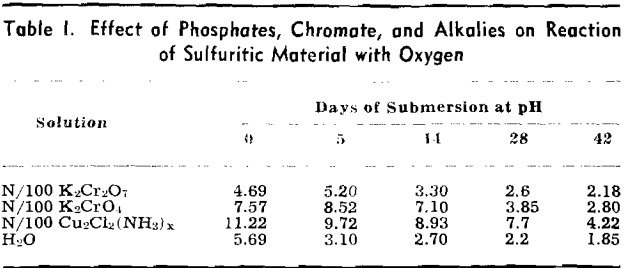
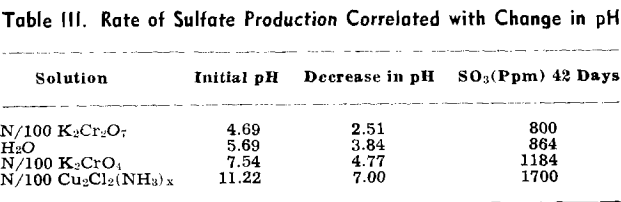
The pH of the solution was determined immediately after it was placed on the sulfuritic material in the flask. The flasks were stoppered with cotton to permit free access of air but to keep out dust, and allowed to stand on the laboratory shelves. The pH was taken at intervals and also the SO» produced was determined.
Experiment 2 was repeated with sulfuritic material from another source which was ground to —8 + 40 mesh and after washing the HCl was washed with water until the washings failed to give a qualitative test for iron or sulfate. Separate 50-g samples were submerged in 1 liter of each solution in 2-liter Erlenmeyer flasks, stoppered with cotton plugs, and allowed to stand on the laboratory shelves as in the previous experiment. pH values were determined at varying time intervals and SO3 determinations made.
The difference in oxidation rate of the sulfuritic material associated with the coal measures and pure yellow pyrite has been previously demonstrated.