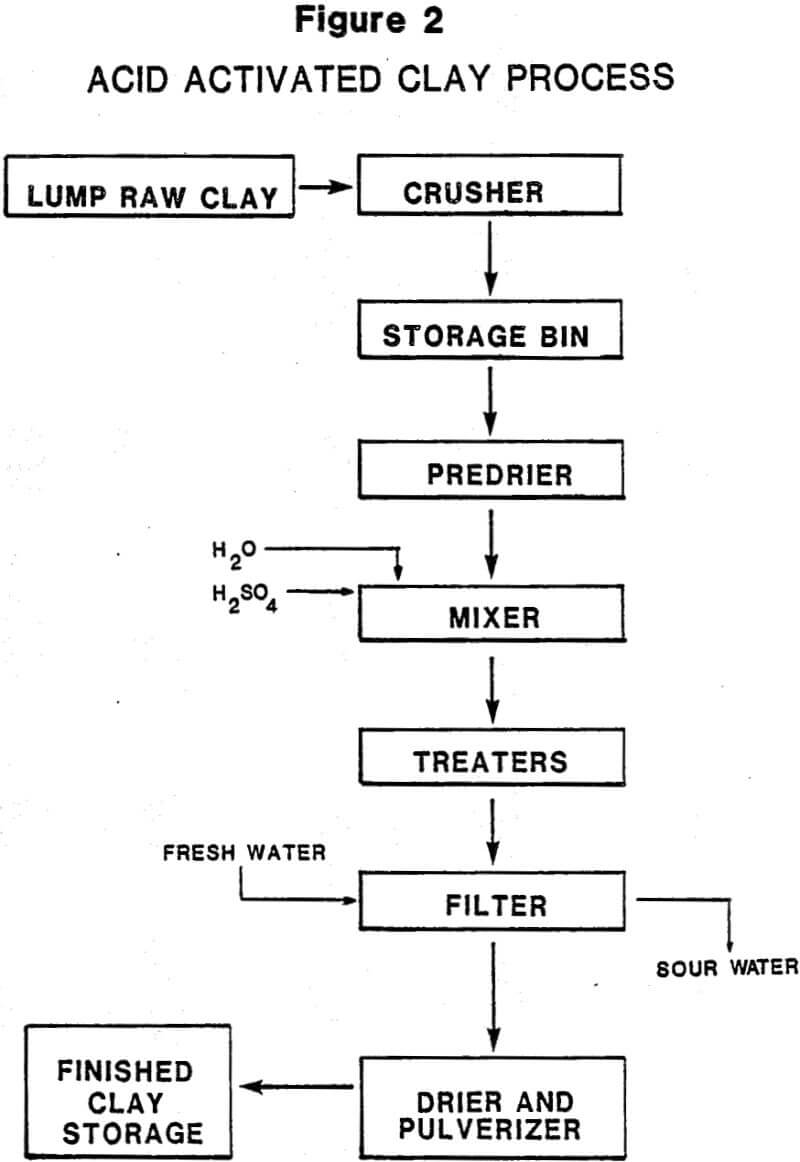
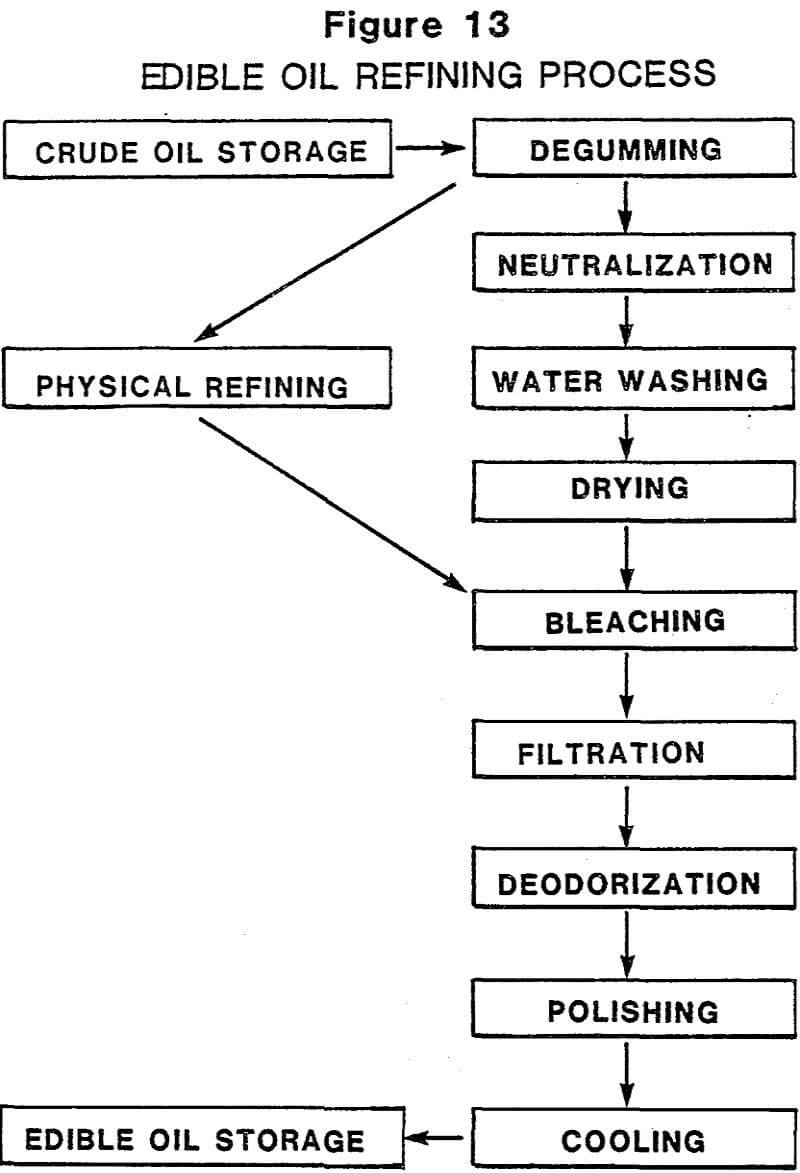
Acid-activated clays are produced almost exclusively from southern or nonswelling bentonites by treatment with various mineral acids. When used for their ability to decolorize or “bleach” color pigments from vegetable and petroleum oils, they are called bleaching clays or bleaching earths. Although sometimes confused with fuller’s earths, which are also sometimes called bleaching clays (Table 1), they differ by virtue of the acid treatment they have received and their significantly higher pigment adsorption efficiencies. Fuller’s earths are used in their native state, processed only by drying and grinding.
It should be emphasized that the acid activation process really only works well when selected bentonites are used. Other clays such as kaolin, attapulgite, talc, etc., improve only slightly or not at all when they are treated with acids. Perhaps even more surprising is the fact that western or swelling bentonites typically will achieve only one-fourth to one-half the activity of selected southern bentonites after acid treatment even though they are often quite similar in chemistry. The reasons for this sensitivity to bentonite source are complex, subtle, and at present only partly understood.
Montmorillonite Structure
In order to understand the chemistry of acid-activated montmorillonite, it is necessary to know something about its crystalline structure. As shown in Figure 1, montmorillonite, a member of the smectite clay group, is a three-layered structure. It is composed of two sheets of silicate tetrahedra (silicon ion surrounded by four oxide ions) linked through shared oxide ions and sandwiched above and below a central sheet of aluminate octahedra (aluminum ion surrounded by four oxide ions and two hydroxide ions) which likewise share oxide bonds with adjacent silicate tetrahedra. The outer layers of silicate tetrahedra are referred to as the tetrahedral layers and the central layer of aluminate octahedra as the octahedral layer. On occasion, a divalent (+2) magnesium (or ferrous) ion substitutes for one of the trivalent (+3) aluminum ions in the octahedral layer. When enough of this substitution occurs (about one magnesium ion for every five aluminum ions), it yields the mineral montmorillonite. The most important consequence, as shown by the 2-dimensional representation of Figure 1, is that each magnesium substitution generates one unit of negative charge on the layer. Since electronic neutrality must be maintained, one unit of positive charge in the form of a mobile, hydrated alkali cation positions itself between the clay layers for each magnesium ion in the octahedral layer.
Because the density of negative layer charge generated is relatively modest in. the case of montmorillonite, the attraction between the layers and the interlayer cations is fairly weak. Under these conditions, interlayer cations are free to move in and out with relative ease (i.e. they are exchangeable if other cations are present to take their place); also the layers themselves can move apart (expand). Only the smectite minerals (such as montmorillonite) exhibit the property of having expandable layers; other 2:1 clay minerals such as mica and vermiculite, talc and pyrophyllite do not expand due to their excessively high layer charge (mica, -vermiculite) or complete lack of it (talc, pyrophyllite).
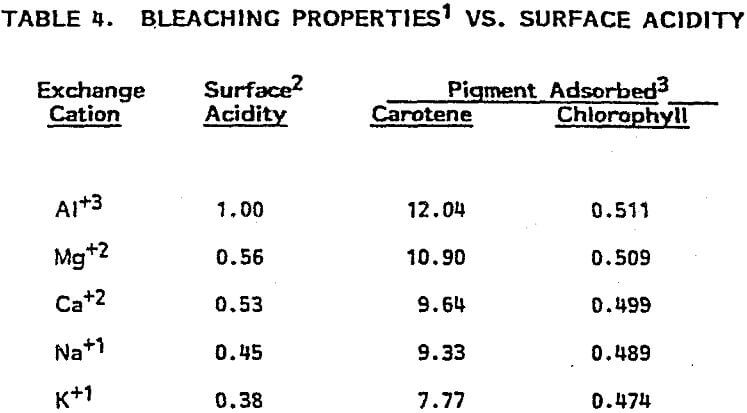
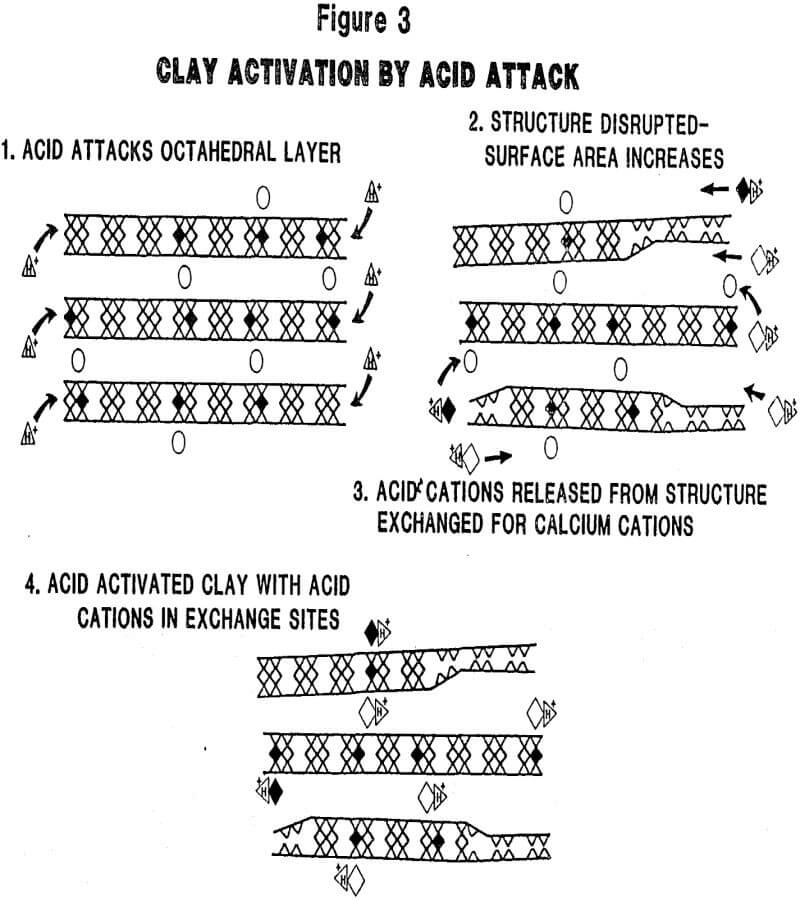
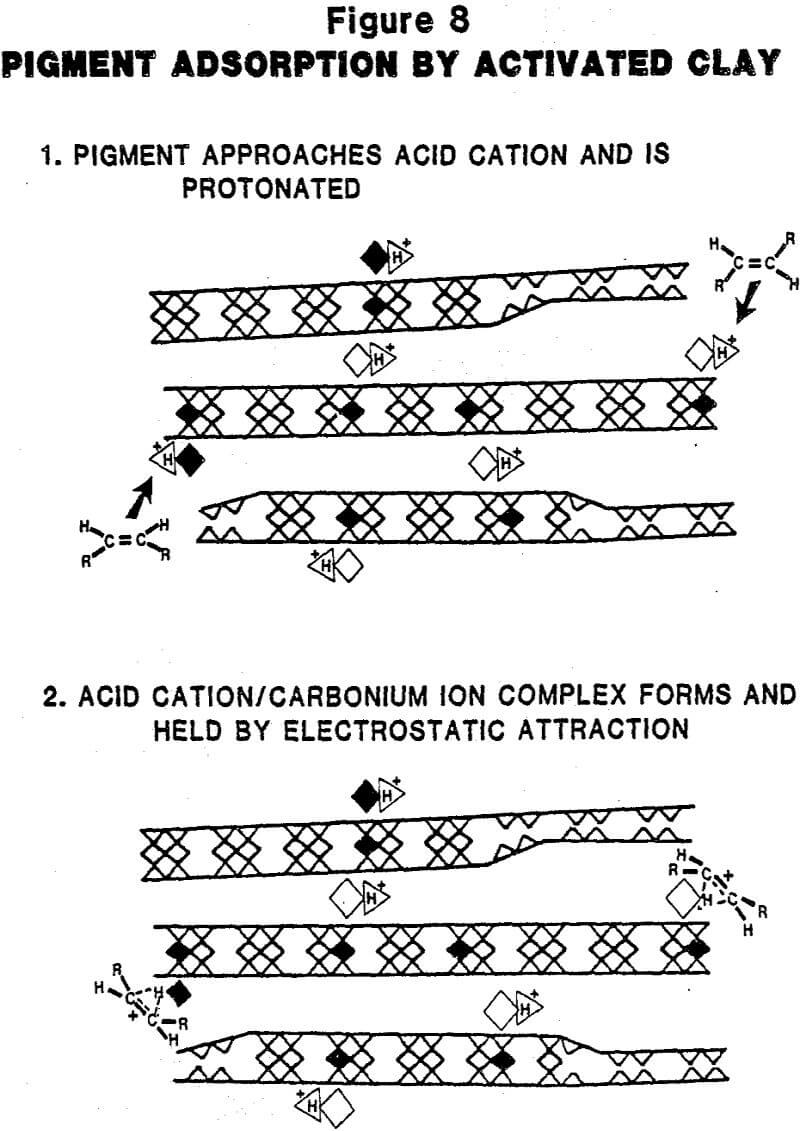
Mechanism of Activation: The mobility of the cations in the montmorillonite structure (vide supra) and the susceptibility of structural ions in the octahedral layer to acid attack play key roles in the acid activation process. As shown in Figure 3, protons from the mineral acid attack the clay structure and liberate structural ions (Al³+, Mg+² Fe+²) from the octahedral layer. Evidence that the clay structure is being attacked (and disrupted) by dissolution of these structural ions is obvious from an examination of the XRD patterns shown in Figure 4 for a series of samples from the same source which have been activated at increasing severity. The loss of pattern indicates a corresponding loss of crystallinity (structure). Two important consequences are: 1) surface area is substantially increased and, 2) alkali and alkaline earth interlayer cations (Ca+², Na+, K+) are exchanged by (more) acidic cations (Al+³, Mg+², Fe+²) from the leach solution.
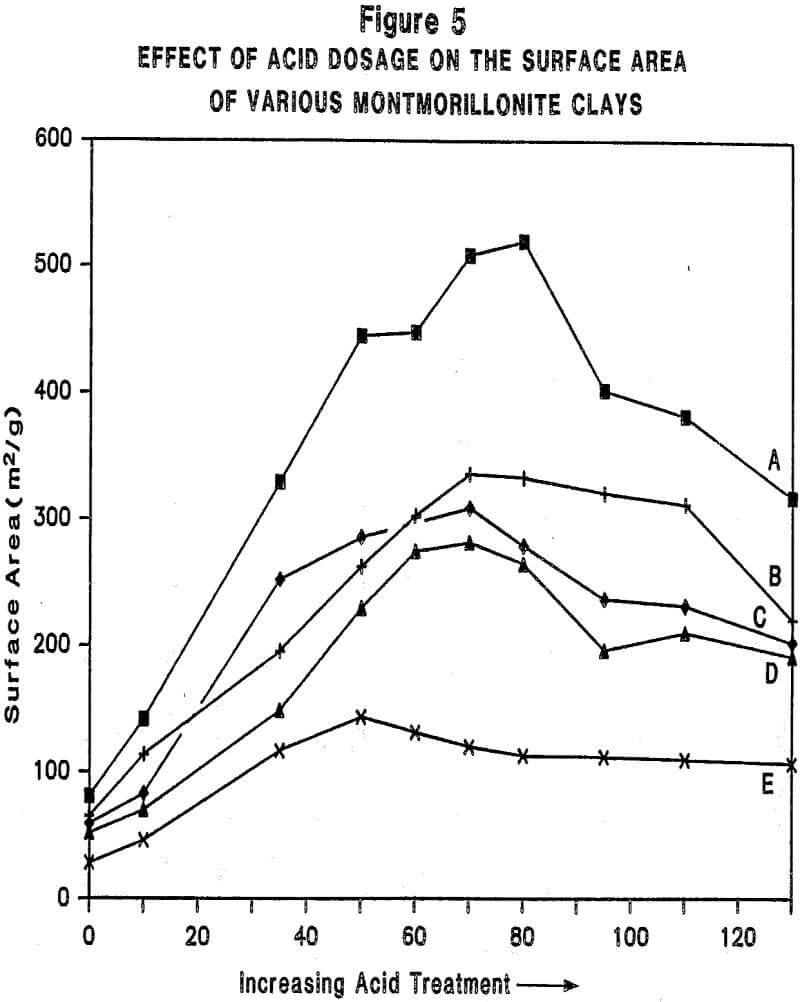
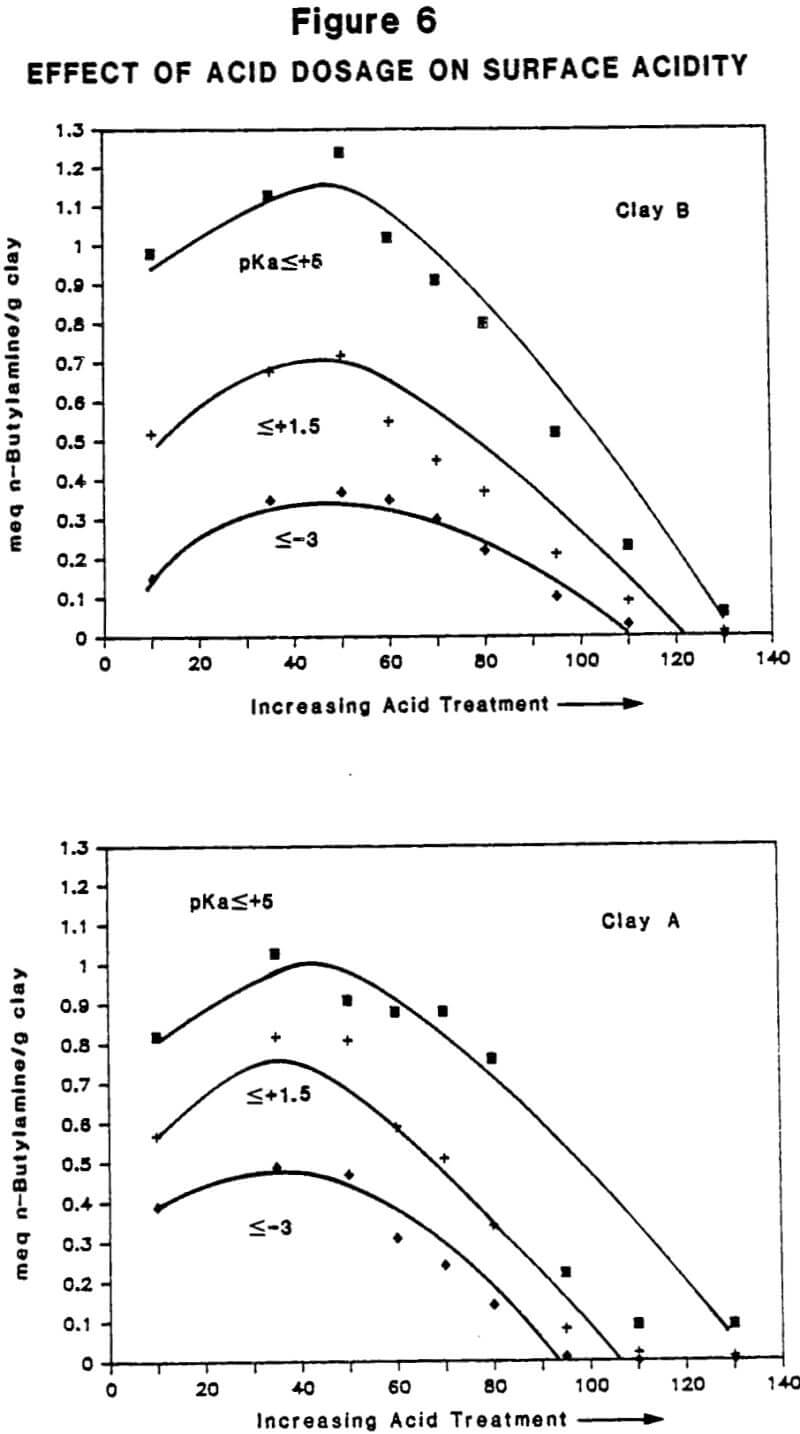
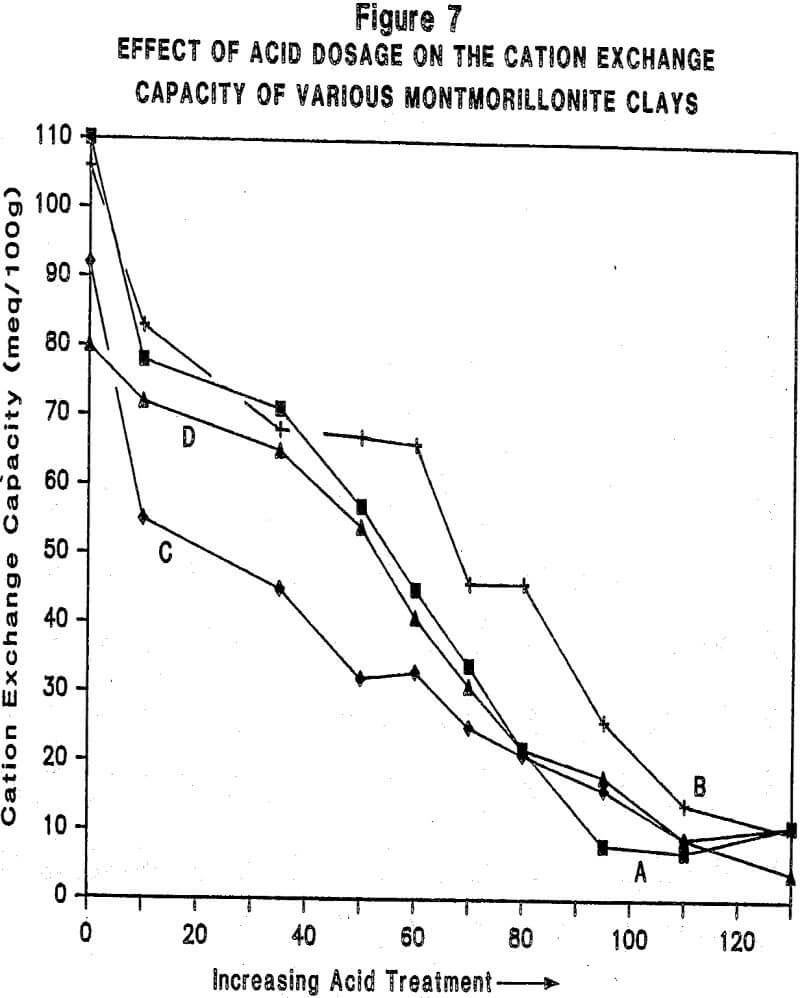
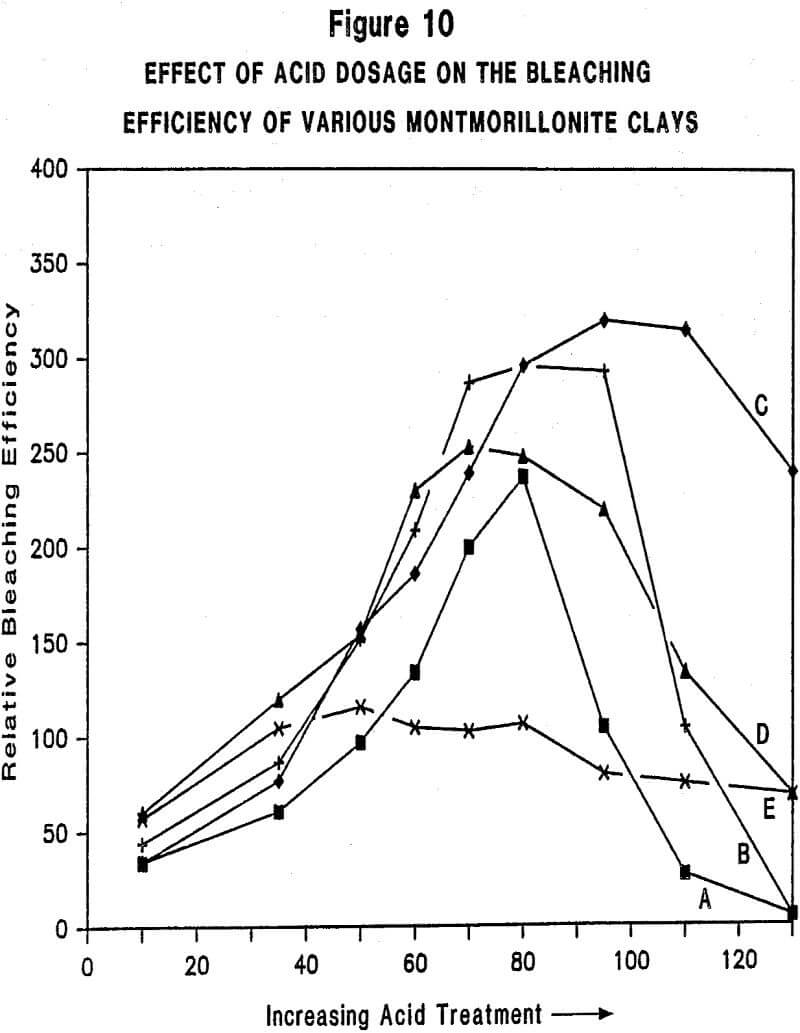
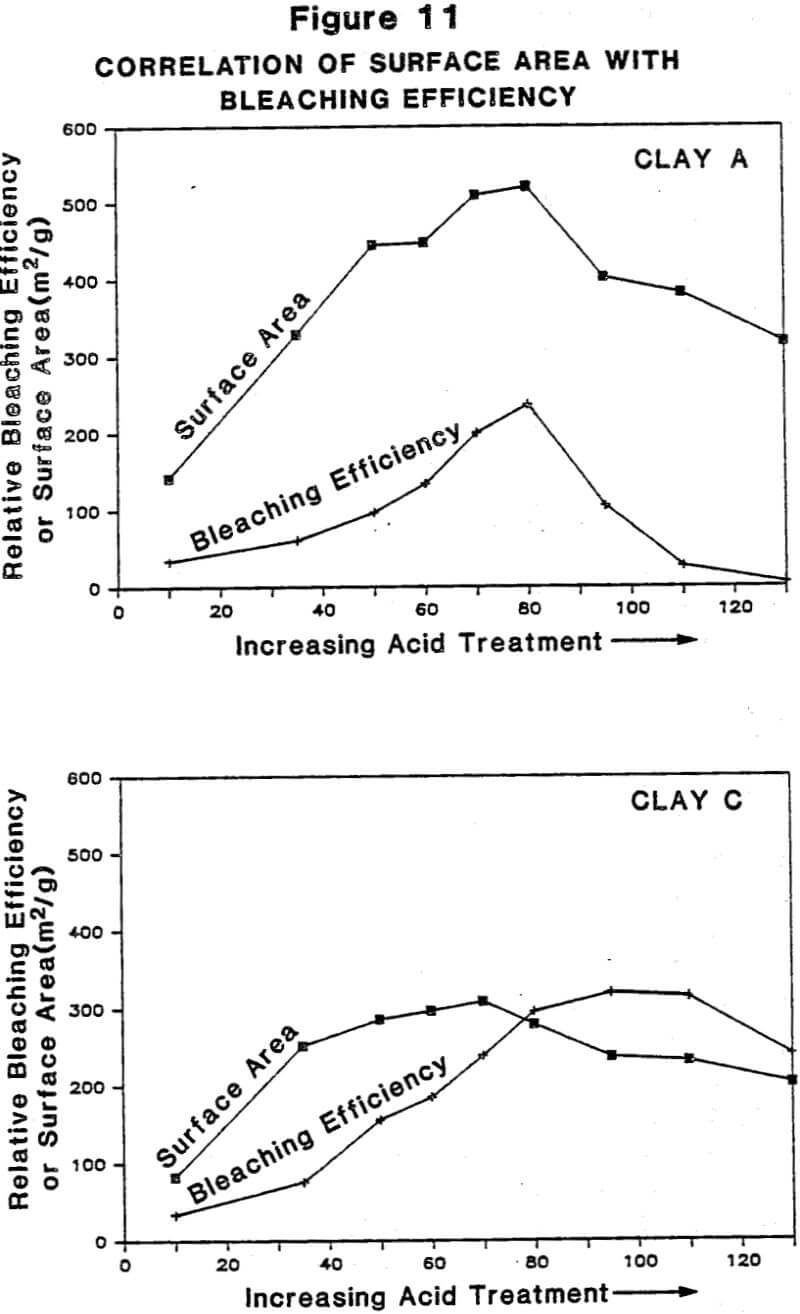
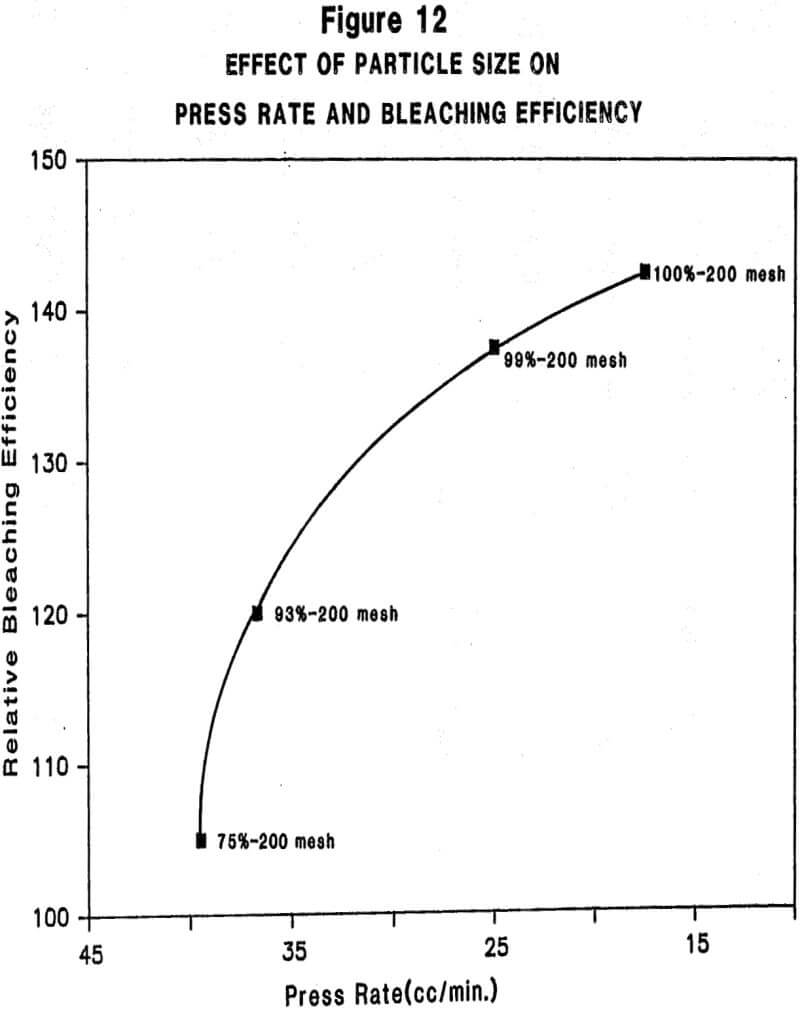
Relationship Between Physiochemical Properties of Acid-Activated Clays and Degree of Activation: Figures 5, 6, and 7 show, respectively, how surface area, surface acidity, and cation exchange capacity change as the severity of acid treatment is increased for a number of bentonite samples. Clays A, B, C, D are nonswelling bentonltes. Clay E is a swelling bentonite (Clay Spur) of the western type and, as is typical, changes relatively little upon acid activation. As is obvious, surface area can be made to improve quite dramatically for the non-swelling bentonltes, but in all cases it does pass through a maximum beyond which additional acid treatment actually reduces surface area. Other workers have reported similar results. Surface area is expected to be important on the basis of theoretical considerations because adsorption and catalysis should increase with Increasing surface area. It is also worth noting that each particular bentonite responds individually with respect to the maximum surface area it achieves and the acid requirements to reach that maximum. This Illustrates the fact that each bentonite deposit is somewhat different, and requires that each be examined individually to determine how best to optimize its properties.
Surface acidity, as shown in Figure 6, measures both the number of acidic sites on the clay surface and their relative strength. For reasons discussed below, surface acidity is an important determinant of performance when acid- activated clays are used as adsorbents or catalysts. Surface acidity has been determined in this case by base titration using Hammett indicators of known pKa. The pKa value quantifies the strength of the acid sites, the lower number denoting higher acid strength. Acid sites possessing a pKa value of -3 are approximately as strong as sulfuric acid; those possessing a pKa value of 5 are considered weak sites, with the approximate strength of acetic acid. As with surface area, surface acidity also passes through a maximum with increasing acid treatment. The trend also shows that it is possible to over-activate a clay and lose surface acidity entirely. This is one reason why adsorption/catalysis is drastically reduced at the upper levels of activation.
Cation exchange capacity measures the ability of clay minerals to exchange their interlayer (mobile) cations for other cations; for acid-activated clays, it gives an indication of the extent to which cation sites are destroyed by the activation process. The number of cation sites and the cations associated with these sites are known to be important to the adsorptive and catalytic properties of acid-activated clays. What is apparent from an examination of Figure 7 is that cation sites (and cations) decrease with increasing acid treatment.
In summary then, acid activation causes both surface area and surface acidity to increase to maxima while cation exchange capacity (number of sites) decreases with increasing severity of treatment.