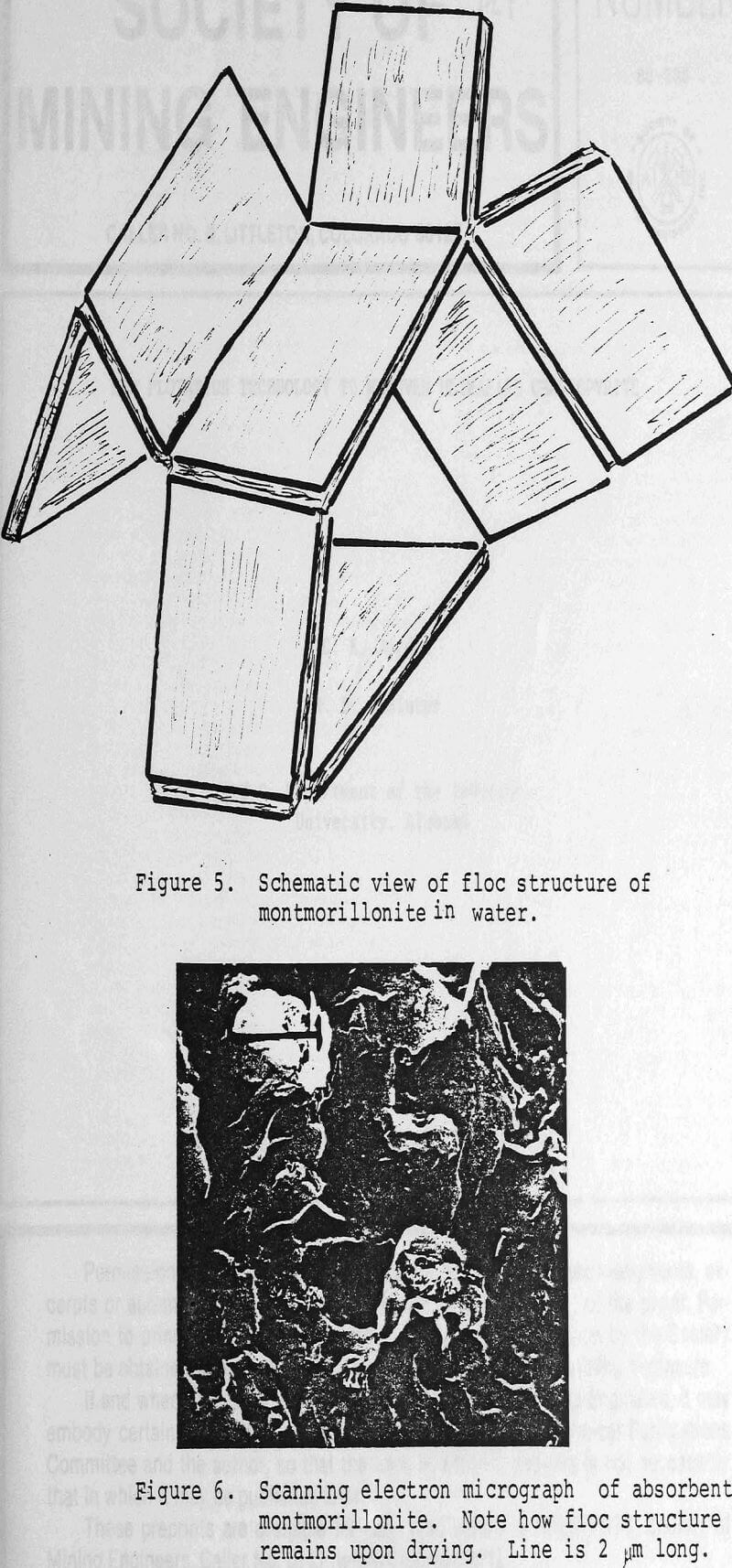
Key Properties and Tests of Absorbent Clays: Clay deposits in the ground contain from 30-40% water. This leads to the remarkable observation that a thick clay bed that can support heavy machinery is by volume 50% water. Most clays when they dry shrink noticeably. Wyoming bentonite, for example, develops a “popcorn-like” appearance on drying owing to a great reduction in volume. Other clays develop numerous fissures and spall apart. Absorbent clays, conversely, show relatively little volume change on drying. The open but firm texture of clay particles in the wet state remains intact after the departure of the water. Also, the voids among the particles interconnect, so the water leaves without disrupting the texture. These voids now are available to reabsorb fluids such as oil or water.
Absorbent minerals clearly must absorb liquids readily and rapidly. Techniques to measure the maximum absorption involve pouring a liquid, usually oil or water, through a column of granules, or immersing a wire cone containing granules into the liquid. Another measurement, the liquid holding capacity, is of great interest to the agricultural carriers industry. This measures the amount of liquid the granules can absorb without sticking together. The measurement involves adding monochlorobenzene, or other surrogate for the agricultural chemical, into a jar containing the granules. The jar is capped and shaken, then the test is repeated until the granules stick together. Typical liquid holding capacities range from 25 weight percent to 35% percent.
The granules must not break down excessively in normal shipment and use. The standard test involves shaking granules on a screen with steel balls in a RoTap sieve shaker. The amount of granules surviving this ordeal then is measured. The test is perhaps too harsh, but does give some important information on the relative hardness. An excessively soft agricultural granule, for example, could produce undesirable amounts of dust containing toxic chemicals.
Absorbent clays must have relatively low bulk densities. Materials with high bulk densities are difficult to handle, and have insufficient internal void spaces to provide absorptivity. Typical values range from 400 kg/m³ for diatomite to up to 720 kg/m³ for absorbent montmorillonites. In comparison, drilling-fluid grade montmorillonite has a bulk density of 960 kg/m³. Several methods exist to measure bulk density, but the Ohaus method is the most reproducible.
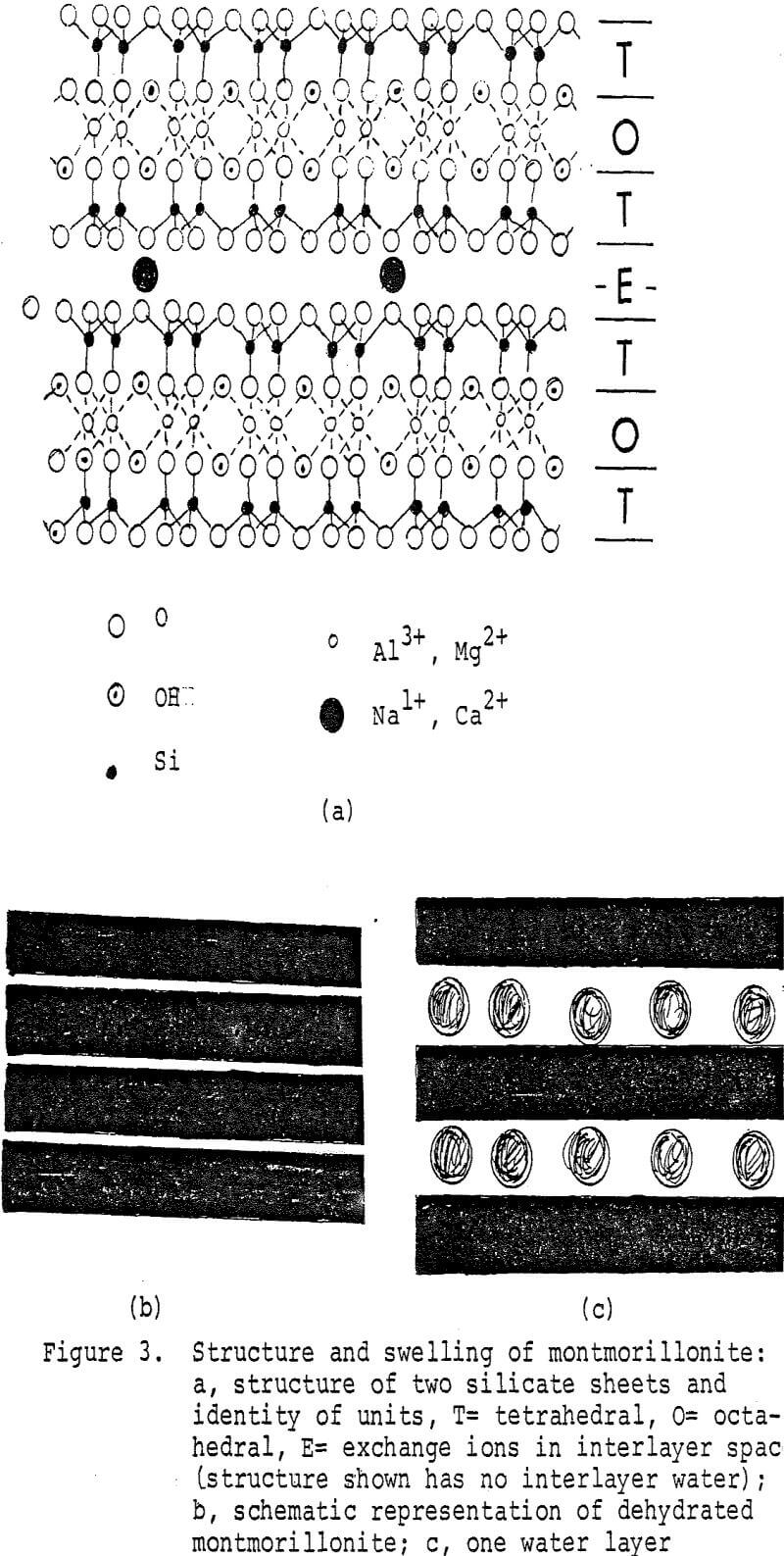
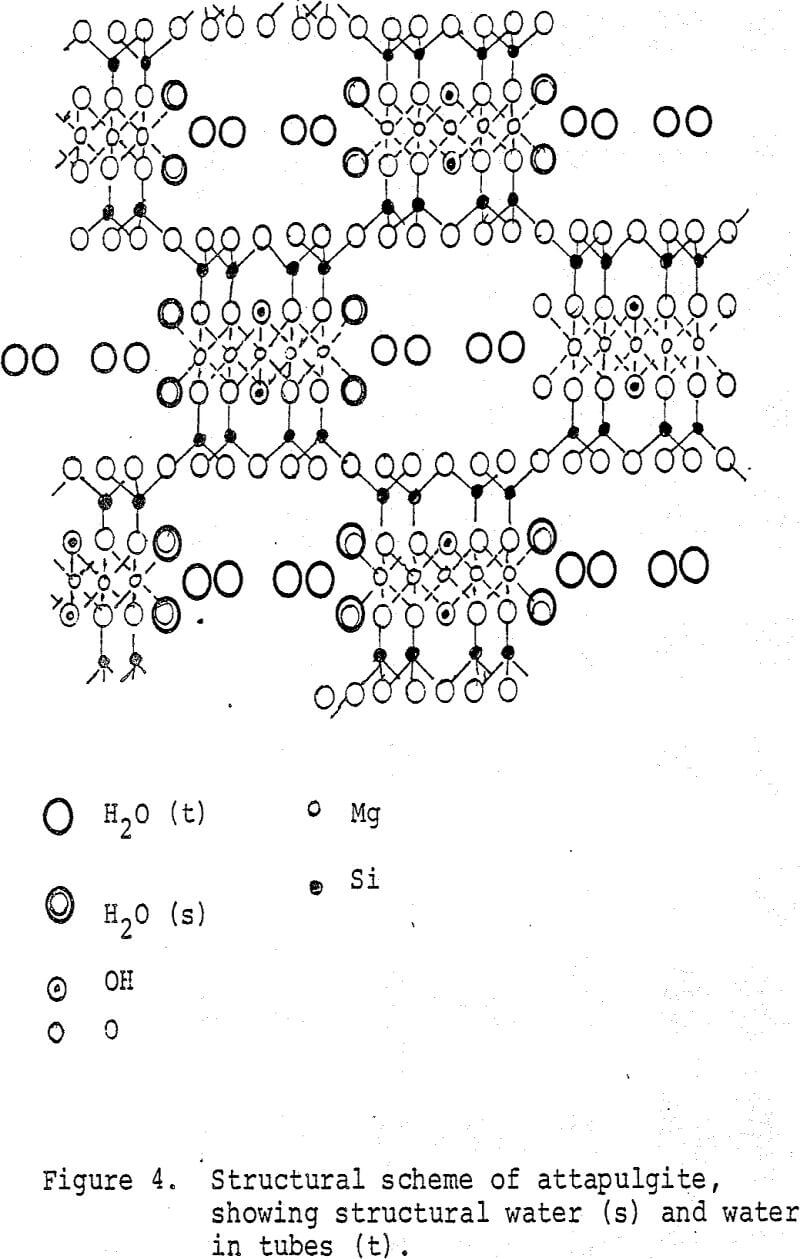
Structure of the Silicates: A full understanding of these properties, especially for montmorillonite and attapulgite, necessitates an understanding of their crystal structure. These clay minerals have seemingly complex structures. Fortunately, these structures arise from combinations of only two geometric shapes, the tetrahedron and the octahedron (Figure 2). The tetrahedron, a 4-sided solid, consists of a central cation surrounded by 4 anions on the apices. The octahedron, an 8-sided solid, consists of a central cation surrounded by 6 anions on the apices. A sheet of tetrahedra would result by sharing corners. A sheet of octahedra would result by sharing edges. In the clay minerals, the tetrahedral sheet consists basically of internal Si cations surrounded by O²- anions. The octahedral sheet consists of either Mg²+ cations surrounded by O²- and (OH)¹- anions or Al + cations and vaciences surrounded by the same anions.
Careful examination of the distances between the apices of the tetrahedra and the octahedra reveal some are virtually identical. The common anions of these apices can be shared between the tetrahedra and octahedra. Actual crystal structures result from this sharing of structural elements. If the tetrahedral sheet is superimposed on one octahedral sheet containing Al³+ then kaolinite (china clay) results. The combined sheets, 0.715 nm in thickness, can stack one atop another. If two tetrahedral sheets sandwich an octahedral sheet containing Al³+, then the mineral pyrophyllite results. The combined sheets are 0.92 nm thick. They are stacked one atop another to form a crystal. Only van der Waals attraction hold the sheets together. Consequently, pyrophyllite is very soft.
In crystal chemistry, ionic size is as important as ionic valence. For example, Mg²+ (ionic radius 0.066 nm) can substitute for Al (ionic radius 0.051 nm). This can occur in the pyrophyllite-like structure to a limited extent. This is the mineral montmorillonite. Crystals must be electrically neutral. Substitution of a lower valence ion, however, for a higher valence ion will result in an overall negative charge on the crystal, an intolerable situation. As the crystal of montmorillonite grows, it will absorb from the surrounding solution cations such as Na¹+ and Ca²+ to balance the charge. These cations, known as “exchange cations” are located principally on the planar sheets. A crystal of montmorillonite resembles a stack of playing cards. The tetrahedral-octahedral-tetrahedral sheet forms each card. The exchange cations are located between the cards, or sheets. The crystals are sub-rectangular or irregular sheets that do not grow very large. They rarely exceed one nm in lateral extent. Only with an electron microscope can the crystals be seen. This is why the clay appears non-crystalline.