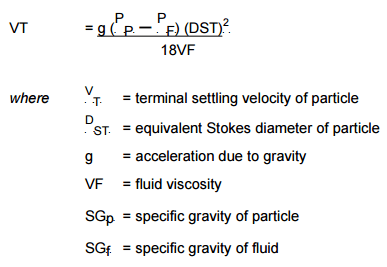
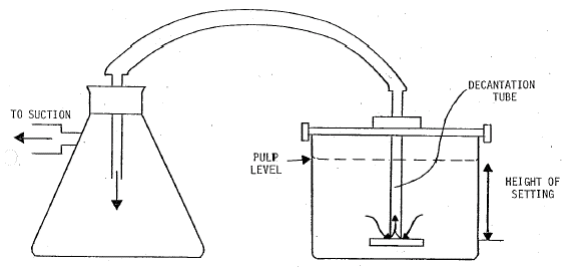
This method for Beaker decantation is a technique used to separate a mineral sample into two size fractions according to the differences in settling velocities of the particles. It is a technique which can be used to accurately split a sample at a pre-determined cut size but has some disadvantages in that it is a time consuming method especially when splitting at fine sizes.
Also a dilute solids content is required to stop natural coagulation from occurring. A spherical shape is assumed for the calculations.
The procedure is based on Stokes Law which relates the settling velocity of a particle to its size and specific gravity through the equation.
By calculating the settling velocity of a particle of the required cut size and specific gravity, the time for the particle to fall a certain distance unhindered through the liquid, can be calculated.
Beaker Decantation Method/Procedure
- Select/choose cut size
- Choose specific gravity of mineral
- Measure temperature of fluid and determine value of viscosity of that fluid.
- Determine specific gravity of fluid.
Equipment Required
A settling vessel is required, the size of which depends on the amount of sample and the cut size chosen. The vessel should be large enough to avoid any form of coagulation which would interfere with the unhindered settling of the particles. The higher the settling height the longer the settling time – sometimes several hours.
A syphon to remove the fluid containing the unsettled particles and decantation equipment, usually consisting of a plate with a tube attached with facilities to set at a predetermined depth. Decant fluid can then pass over the plate and up the tube.
Enclose entire system in a water bath to maintain constant temperature and viscosity and reduce disturbances to the sedimentation process, if complete accuracy is required.
Test Procedure
- Place the sample in the sedimentation vessel and fill with water up to the required level. If the sample was previously dry, complete rewetting of the sample must be performed. Ultrasonics may help in the wetting process. To ensure sample does not naturally coagulate, dispersants should be used, but only if no further processing of products is to be performed. If natural coagulation does occur, more dilution may be required.
- Check that all the decanting equipment is ready with the tube set at the correct height, but do not leave the tube in the liquid during the settling period.
- Measure water temperature (to calculate viscosity of fluid) and maintain as constant as possible.
- Agitate the vessel.
- Commence settling time at completion of agitation.
- Position the decantation tube delicately into position prior to completion of settling.
- Remove the supernatant dispersion as quickly as possible. Do not disturb the settled particles.
- Repeat the above procedure several times until clear supernatant is obtained. The separation efficiency is determined by the number of decantation steps.
- Collect all the supernatant, flocculate, dry and weigh to obtain a weight balance.
Method to Classify Particles by Settling
This Beaker Decantation method, which has proved to be reliable and convenient and is describ this procedure as follows:
An amount of sample containing about 100 grams minus 325 mesh material is screened on a 200-mesh screen keeping the volume as small as possible. The minus 200-mesh pulp is then washed onto the 325 screen and the material on the screen rinsed well (200 cc water or so) to remove fine slimes. If the material remaining on the screen is over 15 grams, it is divided into 15-gram portions and each portion treated as follows:
Into a shallow pan, such as a gold pan, run enough water to submerge the bottom of the screen. Lower the screen into this and jiggle so that the water wells up through the screen. Raise and tap the screen. Continue to do this for 5 min. and at the same time keep swishing the sand around on the screen so that it covers the screen evenly. Dry and weigh the plus 200 and the plus 325. This gives a 44-micron separation.
After 1- or 2-min. settling, the water can be decanted from the gold pan and the residue washed into the main body of minus 325-mesh material. This is then allowed to settle if over 2 liters or if much lime is present. The clear supernatant is decanted, and the thick pulp washed into a 2-liter beaker or battery jar. If the sample has ever been in a lime solution, the safest plan is to dilute up to 2 liters and settle once more to wash out most of the lime.
The pulp is then diluted back to about 2 liters, 0.1 gram Na2C03 added, followed by 1 to 5 cc sirupy Na2Si03 and the pulp stirred for 5 min. with a high-speed stirrer. Remove stirrer, and if the suspension appears to be dispersed, proceed.
Take the temperature, then stir with a rubber-stopper-tipped rod to get as much vertical motion as possible for 10 sec., reverse motion just enough to quench all rotary currents, and note the time.
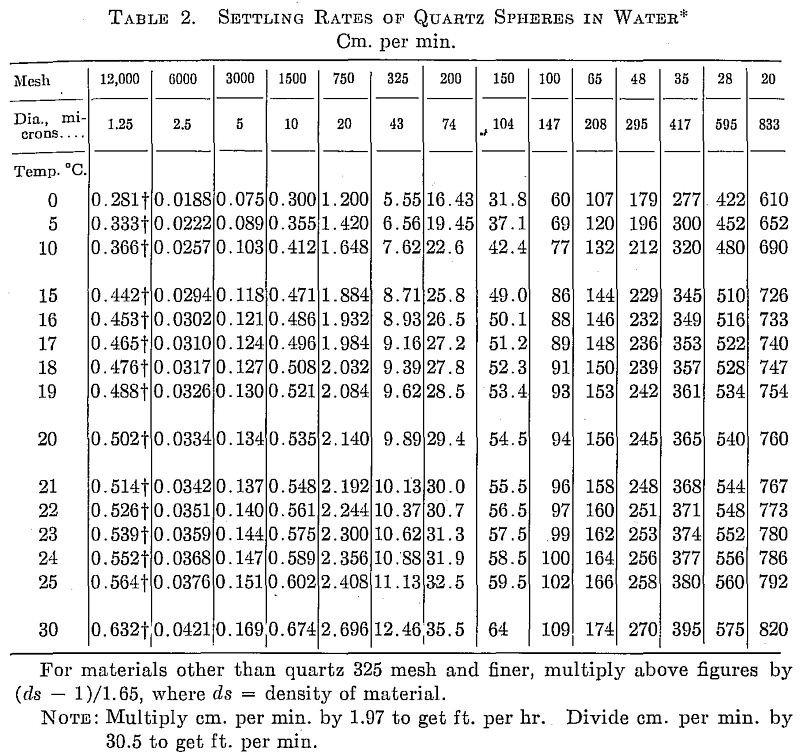
Measure the depth of the suspension from the meniscus to the sand line after tapping. Calculate the time required for a 20-micron particle to settle this distance at the temperature measured by referring to Table 2. Let this = A min. After A min. tap the beaker again, lift carefully, and pour off the supernatant suspension into the other 2-liter beaker. Pour off all the liquid. The +20-micron particles should remain on the bottom of the beaker in a firm cake or at the most only slide a little if the pulp has been properly dispersed.
Time can be started on the 10-micron cut as the suspension is poured above. No stirring is ordinarily necessary.
The +20-micron material is cleaned while the 10-micron cut is standing. A liter of water is added to the residue in the beaker, 1 cc Na2SiO2 added, and the solids thoroughly stirred. Temperature and distance are measured, and the time for a 20-micron particle to settle calculated = A’ min. At the end of A’ min. the suspension is poured off into a 1-liter beaker. This is repeated twice more using the second and third 1-liter beaker to receive the supernatant. These 1-liter beakers are placed in line behind beaker 2 which is full of -20-micron pulp. The residual +20-micron sludge is washed into a dish, decanted, dried, and weighed.
The 10- and 5-micron cuts are made in exactly the same manner as the 20, the supernatant being poured off at the calculated times for 10 and 5 microns. The suspension in the first 1-liter beaker is the first wash solution for the 10-micron cut, and it is poured back into the same beaker after the wash, beaker 2 poured on, etc. The wash solutions are suspended thoroughly before pouring onto the sludge so that the beaker drains clean.
The separations may be carried down to 2.5 or even 1.25 microns if desired, although the times become excessive. Or the butting may be stopped at 10 microns.
The overflow from the finest cut is flocculated with Al2(SO4)3 filtered, dried, and weighed.
The percentage of each size is figured on the basis of the total of the weights of the finished samples.
If a 30-, 40-, or 44-micron separation is desired, it is most conveniently made on the finished + 20-micron partial, using four 1-liter portions of water with no dispersant. The supernatants are all poured into a common receptacle, settled a half hour; the clear water decanted; and the residue dried and weighed as the +20- micron fraction.
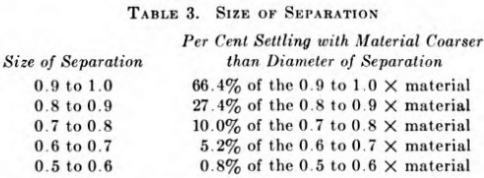
The weight percentages of the micron cuts are reported and plotted at 90 per cent of their nominal diameter; e.g., the per cent in the 20-micron cut is reported as per cent —44 microns +18 microns. The reason for this is that the cuts, even with three washes, are not completely free from finer material but contain enough of it to be just about equivalent to 90 per cent of the diameter. The proportions of the finer sizes contained after four decantations are shown in Table 3.
The rates given in Table 2 are for quartz spheres, density 2.65. As indicated on the table they may be applied to other materials in the fine sizes merely by multiplying by (da — 1) /1.65.
Still other methods of subsieve sizing include use of the sedimentation balance and the Bouyucous hydrometer. These and other related methods are discussed in Micromeritics by J. M. Dali avail e, Pitman, 1943.
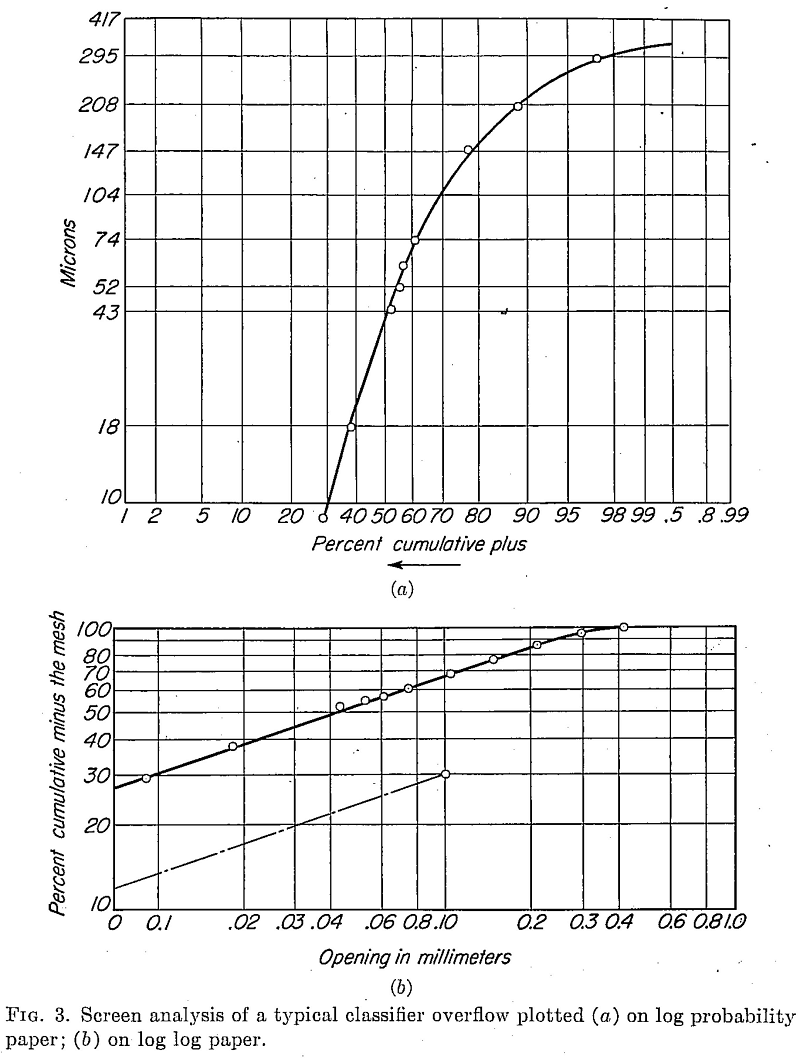
Figures 3a and 3b show two methods of plotting the results of a sizing analysis. The first (Fig. 3a) is a plot on log probability paper (No. 3128, Codex Book Co., Inc., Norwood, Mass.) which, has the advantage of expanding the readings at the coarse end of the distribution line and makes it possible to compute intermediate values throughout the size range quite accurately. In Fig. 3b the same analysis is shown on log log paper with per cent cumulative minus the mesh weight values plotted against millimeter openings. Here the size distribution tends to approach a straight line, and extremely low subsieve readings can be obtained by extrapolation as shown.