Volatilisation of Gold

The boiling point of pure gold has not been determined; calculated according to Wiebe’s formula it would be about 2,240°, or nearly 500° above the melting point of platinum. However, contrary to the belief of the older experimenters, it is sensibly volatile in air at far lower temperatures. Robert Boyle was unaware of this fact, […]
Types of Placers
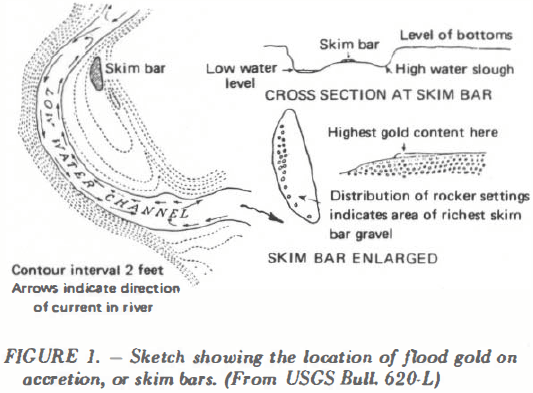
Perhaps the best known schemes for the classification of gold placer deposits are those by Jenkins and Brooks, the former being based on conditions of deposition and the latter on present position of the deposit. The field engineer should acquaint himself with these schemes, particularly that by Jenkins which has been developed in some detail. For […]
Gold Placer Geology
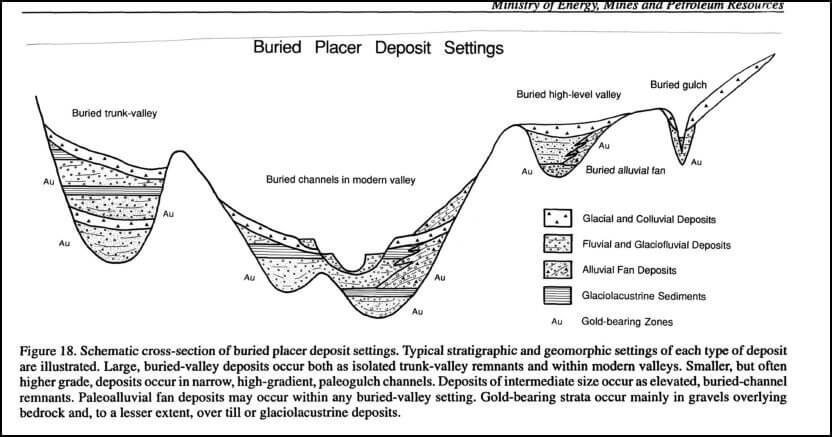
The source of gold or other minerals found in a placer may be one or more of the following: a. Lodes or mineralized zones. b. Erosion of pre-existing placer deposits. c. Low-grade auriferous conglomerates or glacial debris. d. Magmatic segregations and associated basic rocks. e. Regional rocks containing scattered particles of valuable mineral. a. Lodes: Although […]
Colorimetric Analysis
This method of analysis is generally applied to the determination of small percentages of an element or compound, and in this chapter the following will be considered: (a) The Colorimetric Estimation of Copper. (b) The Colorimetric Estimation of Carbon in Steel. And though not coming under this head, (c) The Volumetric Estimation of Copper by […]
ASSAYING CHLORINE BY PRECIPITATION WITH SILVER NITRATE
When we consider the ordinary gravimetric precipitations, it is evident that if the exact strength (as regards precipitation) of the precipitating reagent Apparatus, Reagents.—Apparatus as usual. For the preparation of the standard solution, pure silver nitrate (preferably triple crystallized) is required. For practice in this analysis the student may first estimate the chlorine in pure […]
Analysis by Neutralisation
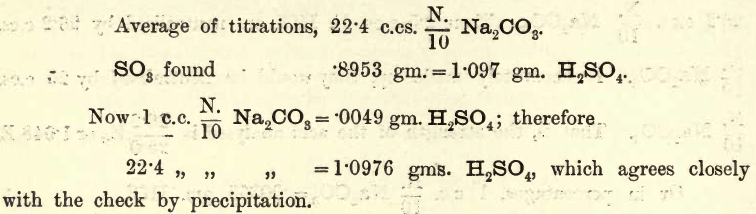
The measurement of the strength of an acid by the quantity of alkali required to neutralise it (termed acidimetry), and the measurement of the strength of an alkali by the quantity of acid required to neutralise it (termed alkalimetry), will be considered in this chapter. The student is asked to determine volumetrically: THE ESTIMATION OF […]
Electrolytic Analysis
The theory of electrolysis cannot be taken up at any length here, and the student desiring fuller information on this subject is referred to the works of Ostwald, Nernst, James Walker, and others. Briefly, it may be stated that on passing a current of electricity through a solution of a salt in water the salt […]
Assaying Lead Ores

To determine by fire-assay the percentage of lead in: (a) The sulphide. Galena (PbS). (b) An oxidised ore containing Cerussite (PbCO3), Anglesite (PbSO4), etc. (a) The sulphide, Galena Methods employed. Regarding the methods employed authorities vary widely ; here two methods are given for sulphide ores, the Soda and Argol (or Nail) method, and the Cyanide method. The […]
What the Effect of Varying Furnace Temperatures

The Wind or Melting Furnace Principle.—According to the equation 2PbO + C = Pb2 + CO2, 446 grains of litharge fused with excess of charcoal should yield 414 grains of metallic lead. If the temperature be too high, PbO volatilises, and this loss, which varies with the temperature and air present, can be checked by […]
Chemical Composition of Cements
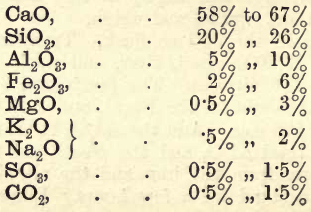
A suitable material for the student to operate on is the well known brand —Portland Cement. As the student has already examined in detail a silicate, the following notes are given somewhat briefly. A good cement should consist chiefly of SiO2 and CaO, with a little Al2O3 and Fe2O3, and less than 2% MgO, and […]
