Analytical Balances Support & Accessories
Brick Pier Support It is essential that the balance, used for analytical work, should be mounted on some firm support; as, not only does the accuracy of the weighings depend upon the freedom of the instrument from vibration and jar, but also the life of the balance itself. It is equally important, also, from the […]
Grinding Mill Power

The power required to drive a tumbling mill is of interest both to the designer and to the mill operator: to the former as a basis of design for the determination of the necessary size of the elements of the machine; and to the latter because all other factors being equal, the most economical machine is […]
Attapulgite Ceramics

The term attapulgite and palygorskite are synonomous. Most mineralogists use the term palygorskite but attapulgite is the preferred industrial term. The structural formula of attapulgite is (OH2) (OH)2, Mg5 si8 O20 · 4H2O. The oxide composition of attapulgite is 53.64% SiO2 8.76% Al2O3, 0.60 TiO2, 3.36% Fe2O3, 0.23% FeO, 2.02% CaO, 9.05% MgO, 10.89% H2O+, 9.12% […]
Native Gold Flotation
The surfaces of native or free gold containing particles are normally hydrophobic and, if the surfaces of such particles are relatively free of contaminants they will have a tendency to float without the addition of a collector. The major sources of contamination on free gold particles are processing chemicals and small particle slime coatings. Gold […]
Ball Mills
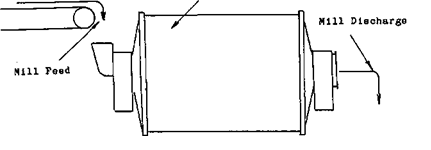
In all ore dressing and milling Operations, including flotation, cyanidation, gravity concentration, and amalgamation, the Working Principle is to crush and grind, often with rod mill or ball mill, the ore in order to liberate the minerals. In the chemical and process industries, grinding is an important step in preparing raw materials for subsequent treatment. […]
Quantitative Chemistry Gravimetric Analysis: PRECIPITATE
WASHING THE PRECIPITATE: A precipitate may be washed directly on the filter, or it may be washed partly by decantation and partly on the filter. If by decantation, the precipitate is allowed to settle, and the supernatant liquid is poured on the filter. Wash water is added to the precipitate, and after settling, the decantation is […]
Gravimetric Analysis: Substance into Solution
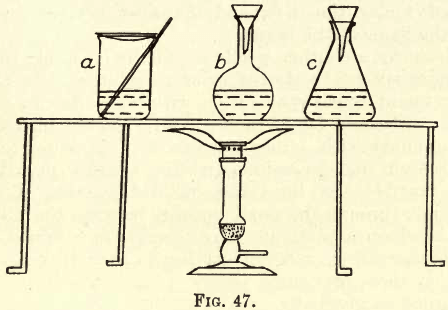
Material of Vessels for Solution The student must consider the effect of the solvent used on the vessel. In most cases the solvent used is an acid or mixture of acids, and for such solvents glass and porcelain are generally used. Platinum may be used, provided no chlorine or other attacking agent be present. (See […]
Analytical Balance
Analytical Balance Principle In this place it will be sufficient to describe the usual chemical balance, designed to carry in each pan a load up to 100 gms. This balance can be obtained at a reasonable figure, and sensitive to 1/10 of a milligram (0.0001 gm.). In the section on Assaying the student will find […]
Mineral Identification by Spectroscopy

FIG. 35 gives an idea of the spectroscope and of its different parts. P is a flint glass prism, having a refracting angle of 60° and resting on a brass plate fixed on a brass support, S. The brass plate carries the collimator tube C, in the end of which nearest to the prism is fixed […]
PREPARATION OF BASE SOLUTIONS
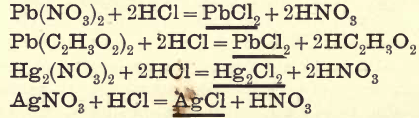
PREPARATION OF SOLUTION FOR BASES 1. Boil the finely-divided substance in distilled water. 2. If insoluble, add ¼ its bulk of strong HCl and boil for two or three minutes. 3. If still insoluble, treat a fresh portion with strong HCl and boil for five minutes ; then add an equal volume of water and warm. The […]
