ASSAY FOR CYANIDE BY TITRATION WITH SILVER NITRATE
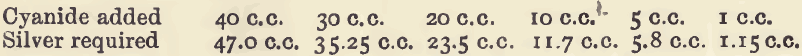
The determination of the quantity of a cyanide is made by finding how much silver nitrate is required to convert the whole of the cyanide into potassium silver cyanide or one of the allied compounds. It will be seen from the equation that 170 parts by weight of silver nitrate are required for 130 parts […]
Gold Parting Process

The thin sheet of metal is dropped into hot dilute nitric acid and boiled for five or six minutes after the brisk action of the acid on the metal has ceased. At this stage nearly all the silver has gone into solution as nitrate of silver and the acid is charged with this salt. This […]
Remove Lead from Gold or Silver
Large quantities of lead carrying gold and silver are sold to refiners in bars weighing about 100 lbs. each. The assay of these alloys presents no special difficulties, but the sampling of them is a question which may be profitably discussed. A molten metal may be conceived to have all the physical states observed in […]
Determine Gold Content By the Microscope

The use of the microscope also is a real advantage in estimating the weights of minute buttons of gold where there is no undue risk in sampling, and where an error of say 1 in 20 on the quantity of gold is tolerable. For ores with copper, lead, zinc, &c., as well as for tailings […]
CUPELLATION & Silver Assaying
The process is as follows:—The cupels, which should have been made some time before and stored in a dry place, are first cleaned by gentle rubbing with the finger and blowing off the loose dust; and then placed in a hot muffle and heated to redness for from 5 to 10 minutes before the alloy […]
Assaying Lead Determination Method Pb

The chief ore of lead is galena, a sulphide of lead, common in most mining districts, and frequently associated with blende and copper-pyrites. It always carries more or less silver; so that in the assay of the ore a silver determination is always necessary. Carbonate (cerussite), sulphate (anglesite), and phosphate (pyromorphite) of lead also occur […]
Assaying Antimony Determination Method
Antimony occurs in the native state, but is rare; its common ore is antimonite, the sulphide (Sb2S8). Jamesonite and other sulphides of lead and antimony are frequently met with. Sulphide of antimony is also a constituent of fahlerz and of many silver ores. Antimonite occurs generally in fibrous masses, has a lead-like metallic lustre, is […]
Assaying Bismuth Determination Method

Bismuth is nearly always found in nature in the metallic state; but occasionally it is met with as sulphide in bismuthine and as carbonate in bismutite. It is also found in some comparatively rare minerals, such as tetradymite, combined with tellurium, and associated with gold. In minute quantities it is widely distributed: it is a […]
Assaying Thallium Determination Method
Thallium is a rare metal, found in small quantities in some varieties of iron and copper pyrites, and in some lithia micas. It resembles lead in appearance. Its compounds resemble the salts of the alkalies in some respects; and, in others, those of the heavy metals. It is detected by the green colour which its […]
Assaying Cadmium Determination Methods
Cadmium occurs in nature as cadmium sulphide in greenockite, CdS, which is very rare. It is widely diffused in calamine, blende, and other zinc ores, forming, in some cases, as much as 2 or 3 per cent, of the ore. Oxide of cadmium forms the “ brown blaze ” of the zinc smelters. Sulphide of […]
