Electromagnetic Separators for Strongly Magnetic Minerals
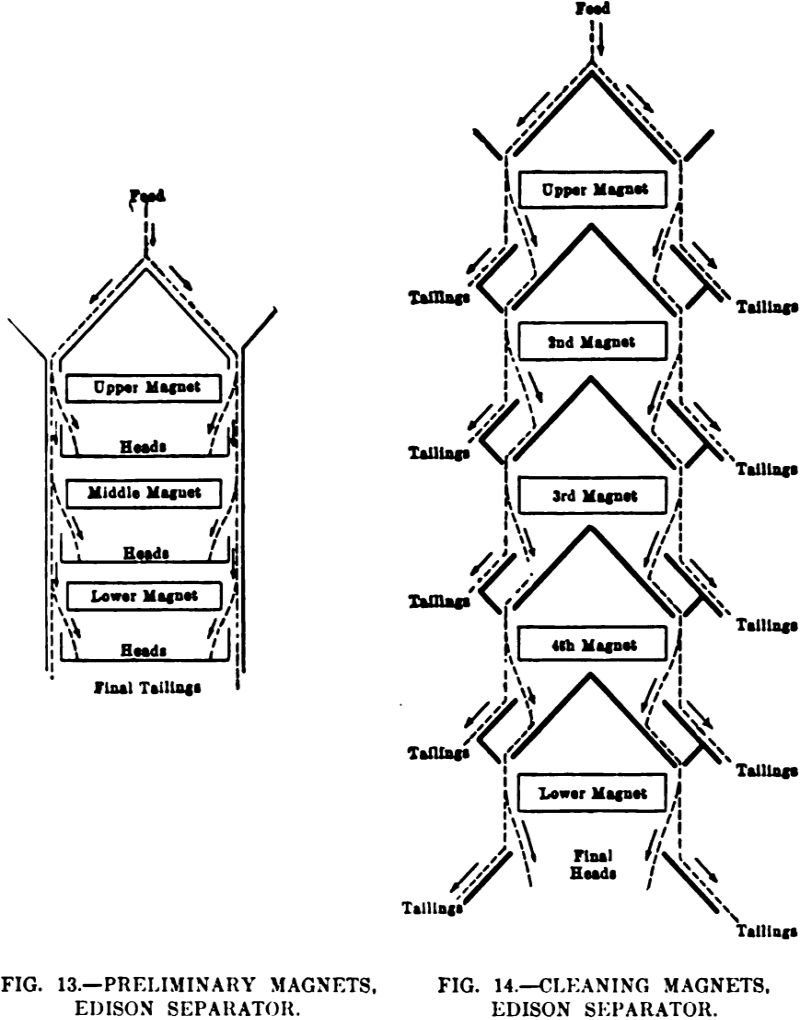
Many classifications based on the method of treatment, on differences in construction, etc., have been suggested to include the different types of magnetic separators; separators with stationary magnets, and those whose magnets revolve; separators in which the ore is attracted directly against the magnet, and those which interpose a nonmagnetic belt or drum between the […]
Indirect Titration
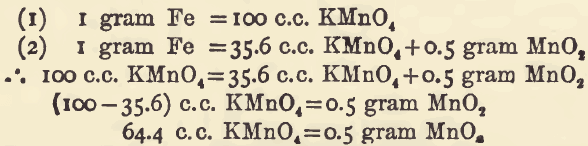
The action of permanganate of potash upon a ferrous solution is one of oxidation, hence it is evident that if any other oxidising agent is present it will count as permanganate. In such a case the titration can be used (indirectly) to estimate the quantity of such oxidising agent, by determining how much less of […]
Titrometric Assays
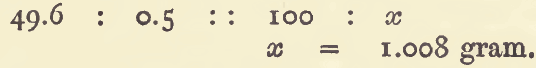
Within the limits of the error of experiment, a definite volume of a solution or gas represents a certain weight of metal or other substance, hence the exact weight may be determined by experiment. The error of experiment may be reduced to insignificant dimensions by repeating the experiment, and taking the mean of three or four […]
Gasometric Assays
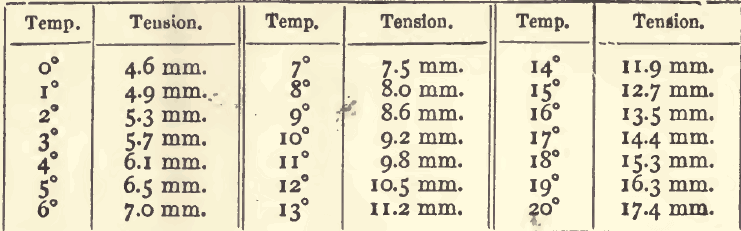
Gasometric methods are not much used by assayers, and, therefore, those students who wish to study them more fully than the limits of this work will permit, are recommended to consult Winkler and Lunge’s text-book on the subject. The methods are without doubt capable of a more extended application. In measuring liquids, ordinary variations of […]
Oxidising Agents used in Assaying
The chief oxidising agents (which are also de-sulphurisers) are the following: Nitre, or Potassic Nitrate.—This salt fuses very easily to a watery liquid. It oxidises most combustible substances with deflagration, and thereby converts sulphides into sulphates, arsenides into arsenates, and most metals into oxides. In the presence of strong bases, such as soda, the whole […]
De-Oxidising Agents used in Assaying
The de-oxidising agents most in use are the following: Charcoal.—Powdered wood charcoal; it contains more or less hygroscopic moisture and about 3 or 4 per cent, of ash. The rest may be considered carbon. Carbon heated with metallic oxides takes the oxygen ; at low temperatures it forms carbon dioxide, and at higher ones, carbon […]
Assaying Iridium Determination Method
Occurs in nature alloyed with osmium as osmiridium or iridosmine, which is “ rather abundant in the auriferous beach sands of Northern California” (Dana). It occurs in bright metallic scales, which do not alloy with lead, and are insoluble in aqua regia. Iridium also occurs in most platinum ores, and forms as much as two […]
Assaying Platinum Determination Method
Platinum occurs in nature in alluvial deposits associated with gold and some rare metals, generally in fine metallic grains, and, occasionally, in nuggets. It is a grey metal with a high specific gravity, 21.5 when pure and about 18.0 in native specimens. It is fusible only at the highest temperature, and is not acted on by […]
Flatting Gold
Small buttons, such as are got in assaying most gold ores, are placed on a polished steel anvil and flattened by one or two blows with a hammer. The flattened discs are heated to dull redness on a clean cupel and are then ready for parting. Somewhat larger buttons may be similarly treated, but they should be annealed (i.e. […]
Inquartation
The method of separating the gold from the silver in gold-silver alloys by boiling with nitric acid does not act equally well in all cases. An alloy half silver half gold, rolled to thin sheet and boiled for half an hour with nitric acid, may still retain more than two-thirds of its silver. An alloy […]
