ASSAYING LEAD BY AMMONIUM MOLYBDATE Alexander’s Method
Apparatus, Reagents.—The usual apparatus. For the standard solution, ammonium molybdate is required [(NH4)2MoO4], or by a simple calculation the heptamolybdate (NH4)6Mo7O24,4H2O which is non-deliquescent, or the trioxide MoO3 dissolved in NH4HO may be used. For an indicator a solution of 1 gm. tannin in 500 c.cs. water is used. For analysis the student may take […]
ASSAYING ZINC BY POTASSIUM FERROCYANIDE Fahlberg’s Method
Apparatus, Reagents.—The usual apparatus. For the preparation of the standard solution pure K4FeC6N6 (free from K6Fe2C12N12) is used, and pure ZnO is employed for checking the standard. As an indicator a solution of uranium acetate is employed. In the preparation of the ore for analysis the following solutions are required :—About 100 c.cs. of a solution of KClO3 crystals in […]
Assaying Manganese
VOLUMETRIC ESTIMATION OF MANGANESE BY POTASSIUM PERMANGANATE. (Volhard’s Method) Aparatus. Reagents.—The usual apparatus. For the standard solution K2Mn2O8 is required. Zinc oxide free from Mn is used for neutralisation. For analysis the student may take a ferro-manganese ore (containing Fe and Mn). Method, Reactions.—If a dilute, neutral or faintly acid solution of a manganese salt […]
Assaying Arsenic
VOLUMETRIC ESTIMATION OF ARSENIC (Pearce’s Method) Apparatus, Reagents —The usual apparatus. For precipitation E. AgNO3 may be used. For the standard solution NH4CNS is used, and as an indicator a saturated solution of iron alum. For the solution of the silver arseniate, pure HNO3 is required. Method, Reactions.—The ore containing the arsenic is fused with a […]
ESTIMATION OF IRON BY POTASSIUM PERMANGANATE
THE ESTIMATION OF IRON BY STANDARD SOLUTION OF POTASSIUM PERMANGANATE (Marguerite’s Method). Apparatus, Reagents.—The usual volumetric apparatus, For standardising the solution soft iron wire such as is used for binding flowers is best suited. This wire contains 99.6% of iron. Pure K2Mn2O8 is required for the standard solution. If in doubt concerning its purity it […]
ESTIMATION OF IRON BY POTASSIUM BICHROMATE SOLUTION
THE ESTIMATION OF IRON BY STANDARD POTASSIUM BICHROMATE SOLUTION (Penny’s Method). Apparatus, Reagents.—For the preparation of the standard solution pure K2Cr2O7 is required, also the iron wire as before, E. SnCl2, 2/5 E. HCl and E. K6Fe2C12N12, freshly prepared and containing no ferrocyanide. A white porcelain plate is used for the indicator tests. For analysis the student […]
DETERMINATION OF CHLORINE IN BLEACHING POWDER BY IODINE
THE DETERMINATION OF CHLORINE IN BLEACHING POWDER BY IODINE AND STANDARD HYPOSULPHITE. Apparatus, Reagents, etc.—As before. The student may take for analysis a sample of ordinary commercial bleaching powder. Method Reactions.—If, to the milky liquid obtained by treating the powder with water, excess of KI be added, and then HC2H3O2 the chlorine is liberated from […]
Estimation of Copper in Ores by Iodide Method
The estimation of Copper in Ores by the Iodide method.—The standard solution is again used, and needs no further checking. The student may take for analysis a sample of copper pyrites, portion of which he may reserve (after reduction and sampling) for subsequent estimation by the Cyanide method. The Analysis.—Powder the sample to pass an […]
Hydrochloric Acid Solution Strength Determination
Apparatus and Reagents.—The usual volumetric and gravimetric apparatus. The student may take for analysis a sample of the ordinary bench reagent 5E. HCl. He will also require about 10 gms. pure NaHCO3, and should test this substance for Cl and SO3. If these are found, place a small plug of cotton-wool in the neck of […]
AMMONIA SOLUTION STRENGTH DETERMINATION
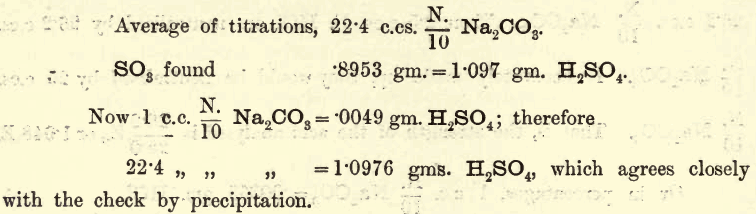
Apparatus, Reagents,—The usual volumetric and gravimetric apparatus. For analysis the student may take the bench reagent labelled 5E. NH4HO. For the standard acid pure H2SO4 of specific gravity 1.840 or thereabouts is most suitable. Pure Na2CO3 (prepared as before) is necessary for standardising the acid. Method, Reactions.—A certain volume of the ammonia solution is taken […]
