Table of Contents
Having some arsenopyrite in your gold ore sample does not insure a full refractory gold processes is needed for recovery. The presence of arsenopyrite and heavy sulphides just tells a component of fraction of the gold extraction process might have a refractory response.
This post is no handbook on procedure for treating sulphide ores for maximum arsenopyrite gold recovery, it is only a real life treatment example of how you can recover gold from arsenopyrite ore. Anytime arsenopyrite is present, you must insure good liberation between Arsenopyrite and your gold.
The mineral fragmentation characteristics of the lithology composites were determined at a nominal grind sizing of 50µm K80. This analysis was conducted on four size fractions using PMA. An overall summary of the mineral fragmentation characteristics is displayed here below.
The Top Chart displays the liberation of arsenopyrite in two dimensions. At the initial nominal primary grind size of 50µm K80, the liberation varied between about 76 and 85 percent, with an average of 82 percent. There was minor interlocking with gangue and pyrite, being, on average, 12 and 5 percent, respectively. At this grind size, liberations would be considered sufficient for good rougher separation.
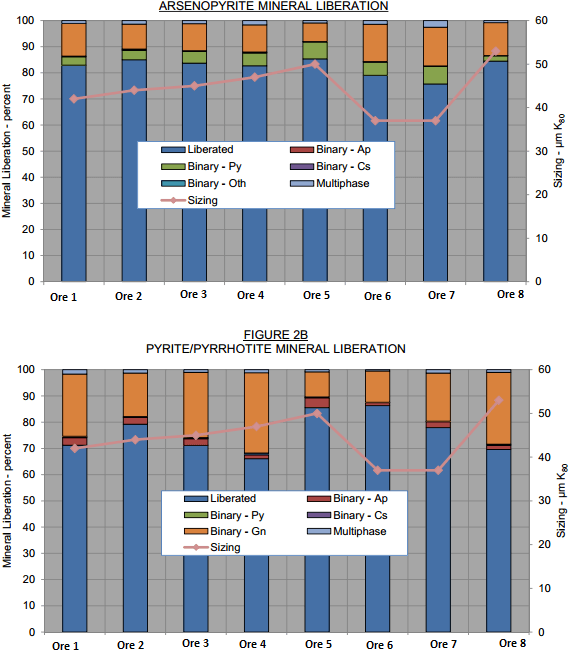
The Chart displays the liberation of pyrite and pyrrhotite. Liberation varied between 66 and 86 percent, with an average of 76 percent. There was a significant amount of interlocking with gangue, being, on average about 20 percent. These binaries contained on average about 50 percent pyrite which should be recoverable under suitable flotation conditions.
How to Recover Gold from Arsenopyrite
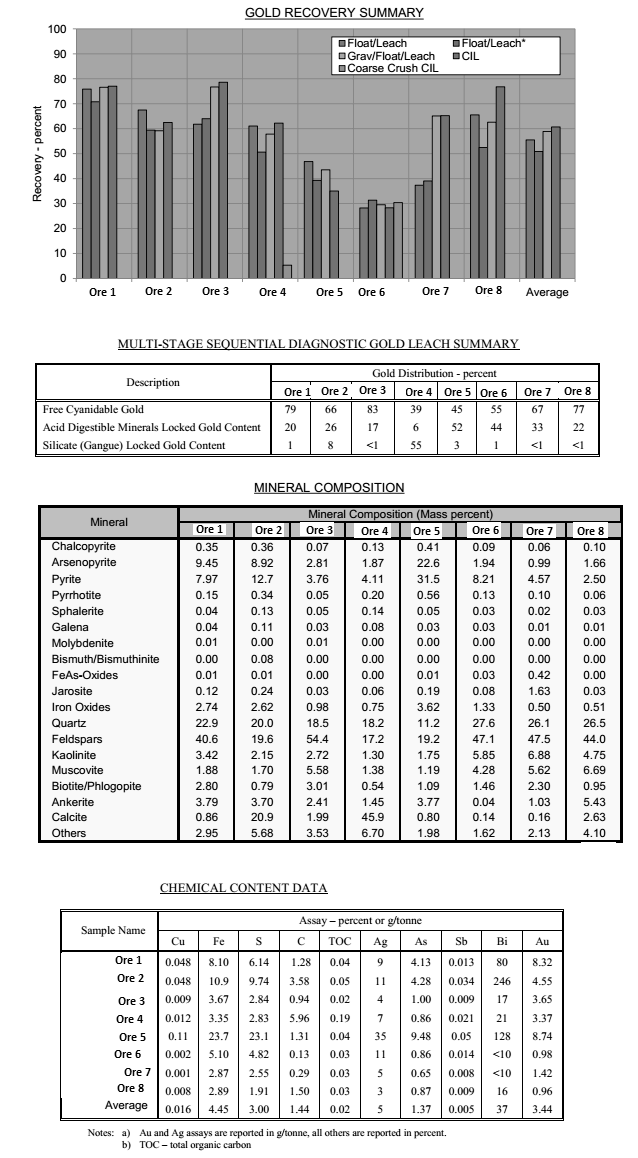
One goal of the Bureau of Mines is to develop technology in increasing productivity and efficiency in processing precious metal resources with minimum degradation to the environment. The purpose of the research reported in this paper was to study the feasibility of recovering gold from select arsenopyrite concentrates by cyanidation and carbon adsorption techniques applicable by the small operator.
The metallurgical treatment of arsenical gold ores is largely dependent on the nature of the gold occurrence in the ore. Gold that occurs with arsenopyrite or pyrite in a silicious gangue is generally in high-grade deposits, limited in size and number, such as occur in the Mother Lode district of California. These deposits contain a wide size range of native gold particles, as well as discrete grains of gold occurring as inclusions in and secretions between the individual crystals of arsenopyrite and pyrite. Some gold may also occur in solid solution in the sulfides.
It is a common practice in processing pyritic gold ores to remove as much gold and sulfide as possible by gravity treatment and/or flotation. Classically, the arsenopyrite concentrate is contacted with mercury to recover the free gold contents. The mercury and amalgam are recovered by washing over an amalgam plate or pan, and the resultant product is retorted to reclaim the mercury and produce a gold sponge residue. The gold sponge is fire-refined to produce a dore bullion. The amalgamation tailings frequently contain significant residual gold values and are shipped to a smelter for further processing.
Another method formerly employed was to roast the arsenopyrite ore or concentrates under oxidizing conditions, and cyanide the calcine to recover the precious metal contents. This process produced enormous quantities of noxious arsenic oxide and sulfur dioxide fumes, which were discharged into the atmosphere, and growing concern about the carcinogenic potential of arsenic fumes (arsenic trioxide) has already made it difficult for producers to sell arsenopyrite concentrates to the smelters. The Environmental Protection Agency and the Occupational Safety and Health Administration plan to impose new standards for arsenic levels in the areas around smelters to protect plant workers and the general population, and the more stringent standards will very likely force the smelters to stop treating arsenopyrite concentrates. Without improved technology, some gold-bearing arsenopyrite-type deposits could be rendered unprofitable despite record high gold prices and demand.
The present investigation is concerned with development of a simple, efficient cyanidation-carbon adsorption procedure for recovering gold and silver from select arsenopyrite concentrates. The proposed method circumvents the problem of arsenic and sulfur emissions and is readily applicable to small operations. The principal advantages of this treatment sequence are rapid and effective gold recovery, efficient loading of the granular activated carbon, and the capability of processing “foul” cyanide solution.
Experimental Procedure and Results
A sample of arsenopyrite table concentrates for the experiments described in this paper was obtained from a small operation in California. Analysis of the concentrates follows:
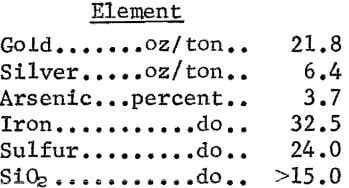
Spectrographic analysis revealed that the concentrate also contained small amounts of aluminum (0.5 pct) , copper (0.02 pct), magnesium (0.3 pct), manganese (0.5 pct), lead (0.2 pct), nickel (0.02 pct), and zinc (0.1 pct). Mineralogical examination by electron microprobe analysis determined the concentrate to be mainly pyrite and arsenopyrite as discrete phases, with lesser amounts pyrrhotite, chalcopyrite, iron oxides (hematite, limonite, and magnetite), and silicates. Based on the observations from the mineralogical examination and the results of amalgamation experiments, approximately two-thirds of the total gold occurs in the concentrate as free and partially locked grains up to 100 µm in diameter. The remaining gold was associated with the arsenopyrite as inclusions in the sulfides, and a small percentage was in solid solution.
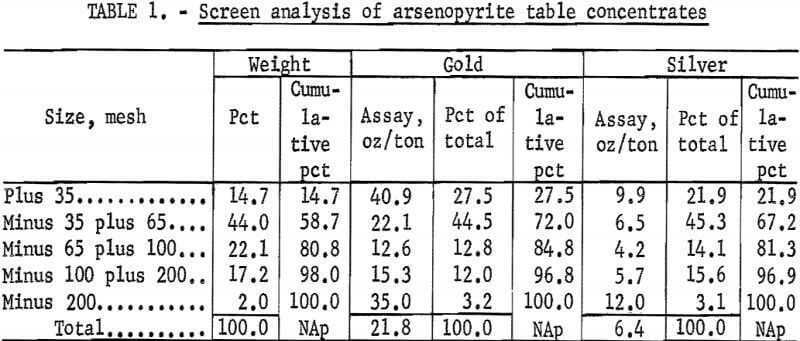
A wet screen analysis of the arsenopyrite concentrates, as received, was done to determine the distribution of precious metals. Fifty-nine percent of the material was plus 65 mesh. The ratio of gold and silver was nearly the same in each size fraction. These data are shown in table 1.
Preliminary Cyanide Leach
Preliminary agitation cyanidation leach experiments were conducted to obtain information about the leachability of the precious metals contained in the arsenopyrite concentrates. Charges of concentrates (200 grams) were agitated for 24 hours in 600 ml of solution containing 10 lb NaCN/ton and lime to maintain protective alkalinity at pH 11.
The pregnant solutions were analyzed for gold and silver by atomic absorption, and the leached residues were analyzed by conventional fire assay methods. Analytical results showed that gold and silver recoveries of 95 and 81 pct, respectively, were obtained in the experiments.
A cyanide leach experiment similar to that mentioned above was conducted to determine the effect of leaching time on gold and silver recovery. A 400- gram charge of concentrate mixed with lime (20 lb CaO/ton of ore) and 1,200 ml of H2O containing 10 lb NaCN/ton of solution were agitated in an open vessel for 96 hours. Gold and silver recovery was determined after each 24 hours of leaching by decanting the pregnant solution and analyzing by the Chiddy method. The solution was replaced with an equal volume of fresh leachant of the same cyanide concentration as that removed, and agitation was resumed. The final leached residue was assayed for precious metal content. The data shown in table 2 indicate that high gold and silver recovery from the arsenopyrite concentrates can be obtained by agitation in cyanide for 72 to 96 hours.
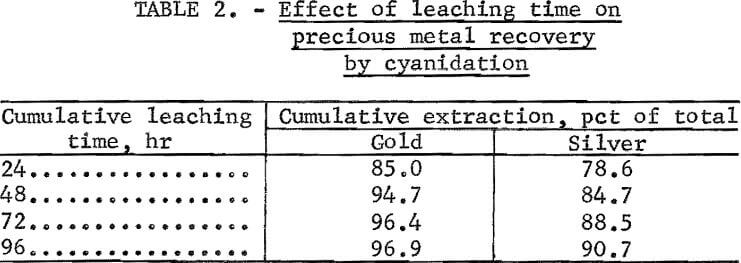
A screen analysis of the leached residue, containing 0.7 oz gold and 0.6 oz silver per ton, was run to determine the distribution of residual precious metal values. Results are shown in table 3.
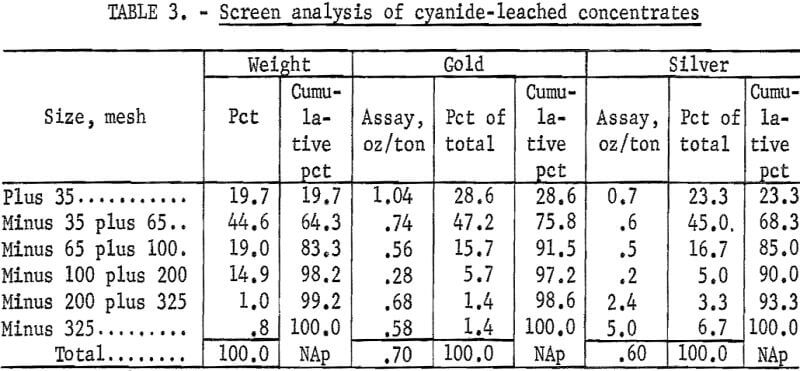
The distribution of gold in the sized fractions indicates that the residual gold values in the tailings are intimately associated with the sulfide minerals and that finer grinding would not significantly improve gold recovery because the gold was leached equally as well from the minus 35-mesh material as from the minus 325-mesh material. The high silver values in the minus 325- mesh fraction indicate that these values occur in a silver-bearing mineral not amenable to cyanidation.
Upflow Percolation Leach
An upflow percolation leach experiment was conducted to determine if the concentrates were amenable to percolation cyanidation leaching. A 2,000-gram charge of concentrate mixed with 20 grams of lime was placed in a 2½-inch- diameter glass column to make a bed about 14 inches deep. The concentrate was leached with a solution containing 6.0 lb NaCN/ton that was pumped upward through the bed at a flow rate of 1.2 l/hr. The pregnant solution overflowing the leach column was collected in a settling tank, and was then passed through a series of five columns, each containing 14.6 grams of minus 6-plus 16-mesh coconut shell activated carbon. The barren solution was recycled to the leach column. Pregnant and barren solution samples were analyzed by atomic absorption for gold and silver content every 6 hours. The pregnant solutions in the first 40 hours of leaching were averaging only 9.5 ppm gold and represented a recovery of only 32 pct of the total gold. In contrast, the total gold recovery from the agitation cyanidation experiments for the same leaching period was 90 pct. The gold dissolution rate in the percolation leach experiment was thought to be slower because insufficient oxygen was available to dissolve the gold according to the following reactions:
4Au + 8CN- + O2 + 2H2O → 4Au (CN)2- + 4 OH-,………………………………….(1)
2Au + 4CN- + O2 + 2H2O → 2Au(CN)2- + H2 O2 + 2OH-……………………….(2)
To test this hypothesis, small amounts of H2 O2 were added to the leach solution to provide oxygen for the dissolution reaction. The daily addition of 1 ml of 30 pct H2O2 per liter of barren solution markedly enhanced the gold dissolution rate without appreciable destruction of the sodium cyanide. The pregnant solutions from the next 20 hours of leaching averaged 31.6 ppm gold and represented an additional 60 pct recovery of the total gold. Gold extractions obtained by percolation leaching for a total of 96 hours were comparable to those obtained by agitation cyanidation. These results indicate that air or oxygen must be injected into the bed of concentrate during leaching if satisfactory recoveries in a reasonable time are to be achieved by the percolation cyanidation method.
Carbon Adsorption Treatment of Pregnant Cyanide Solution
The carbon adsorption study was conducted on 10 liters of pregnant cyanide solution generated by leaching 4,600 grams of table concentrates which was mixed with 20 lb CaO/ton and agitated with 13.8 liters of solution containing 10 lb NaCN/ton. Retention period was 24 hours. The pulp was allowed to settle until the supernatant solution was essentially clear. The supernatant solution was then siphoned from the pulp but not clarified any further. The resulting solution assayed 310 ppm gold and 57 ppm silver, had a pH of 10.7, and contained 2.4 pounds of free NaCN per ton. Significant amounts of sulfide and sulfate sulfur and minor amounts of copper and iron impurities were present. Analyses of the pregnant solution are shown in table 4. The high level of sulfide ion contamination (320 ppm) qualifies the solution to be classified as a “foul preg.”
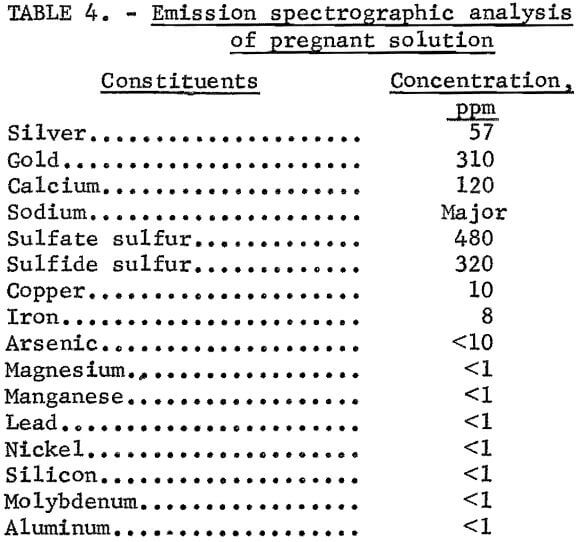
The effect of deleterious substances on cyanidation has been thoroughly studied by other investigators. For example, the sulfide ion severely inhibits the recovery of gold from solution by precipitation of the metal on zinc. Concentration of a few parts per million of sulfide ion inhibits gold cementation appreciably, and at 14.4 ppm sulfide, precipitation of gold on zinc ceases altogether. The mechanism for this detrimental effect by the sulfide ion has not been established, but it is believed to be due to the coating of the zinc metal with zinc sulfide.
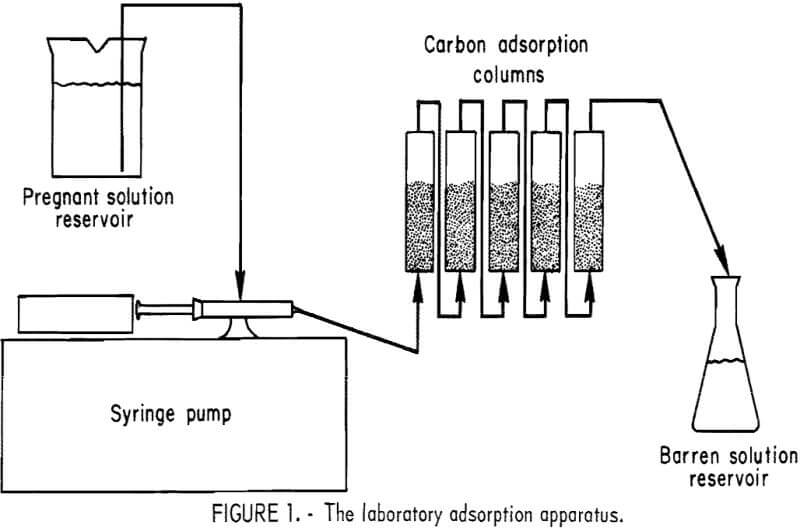
Activated carbon was investigated for gold-silver recovery from the pregnant solutions because of its ability to function with unimpaired efficiency in foul cyanide solutions. Gold tracer (Au195) was added to the gold-silver bearing solutions to monitor the extent of adsorption. The “tagged” pregnant solution was pumped through a series of five adsorption columns, 1 inch in diameter and 4 inches in height, each containing 14.6 grams of minus 6-plus 16-mesh coconut shell activated carbon. Flow rate was 1 l/hr, or 48.5 gal/hr/ft² of cross-sectional area. The laboratory adsorption apparatus used is shown in figure 1. Barren solutions were analyzed for precious metal content, and the carbon in each column was counted for radioactivity after each liter of effluent was collected. The loaded carbons were analyzed by fire assay methods. Results are shown in table 5.
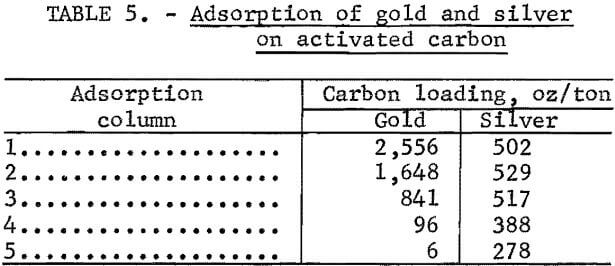
The analysis of the loaded carbon and the measured differences in radioactivity between each loading increment indicated that gold was still being adsorbed in the first carbon column when the entire charge of pregnant solution was consumed. The data indicate that loading on the carbon contained in the first column would have considerably exceeded the 2,556 oz Au/ton achieved if additional pregnant solution had been available.
The initial studies show that granular activated carbon efficiently adsorbed the gold cyanide complex from a foul pregnant leach solution contaminated with sulfide ions. The 2,556-oz loading in the first column was much higher than the 200- to 800-oz loading obtained in general heap-leaching practice, presumably because the feed solution contained 30 to 100 times as much gold as in conventional pregnant solutions generated by treating ores. Barren solutions containing less than 0.1 ppm gold were obtained throughout the test. Silver was also adsorbed but not as efficiently as the gold. Silver loading of about 500 oz/ton of carbon was achieved. No breakthrough of silver was observed at the beginning of the adsorption test; however, after treatment of 4 liters of pregnant solution, trace amounts of silver were detected in the barren solution. The amount of silver increased gradually to 19.5 ppm in the last volume of barren solution.
Cyanidation Carbon Adsorption
Conventional cyanidation-carbon adsorption treatment of an arsenopyrite concentrate can recover 97 pct of the gold and 91 pct of the silver contained in the concentrate. Both agitation and percolation cyanidation are effective in treating this material; however, air injection must be applied in the percolation method to furnish requisite oxygen for efficient dissolution of the gold. The recovery of gold and silver from the generated foul pregnant solutions by carbon adsorption was highly successful. An exceptionally high combined loading of 2,556 oz of gold and 502 oz of silver per ton of carbon was obtained. These loadings are much higher than typical loadings obtained by conventional heap-leaching operations because the precious metal content in the pregnant feed solution is 30 to 100 times greater.
The Bureau of Mines, investigated a cyanidation-carbon adsorption technique for extracting gold from arsenopyrite concentrates. Agitation leach experiments were conducted on 85-pct-minus-35-mesh gravity concentrates containing 21.8 oz gold and 6.4 oz silver per ton. Results obtained in leaching the concentrates showed that 96.9 pct gold and 90.7 pct silver extraction could be achieved in 96 hours of agitation. Gold and silver were recovered from the resulting pregnant solution by exposure to granular activated carbon in a countercurrent system. Carbon loadings of 2,556 oz of gold and 502 oz of silver per ton were achieved. These loadings are significantly higher than heretofore thought practical.
