Thiourea (H2NCSNH2) is an organic compound, the crystals of which dissolve in water to yield an aqueous form stable in acidic solutions. The unique feature of thiourea is that, in aqueous solution it reacts with certain transition metal ions to form stable cationic complexes. For example, the following generalized reaction illustrates the complexing role of thiourea.
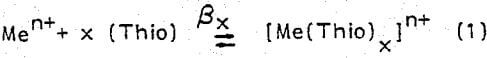
where Me is the metal; n, valence; Thio, represents thiourea; x coordination or stoichiometry number; and βx, overall formation constant.
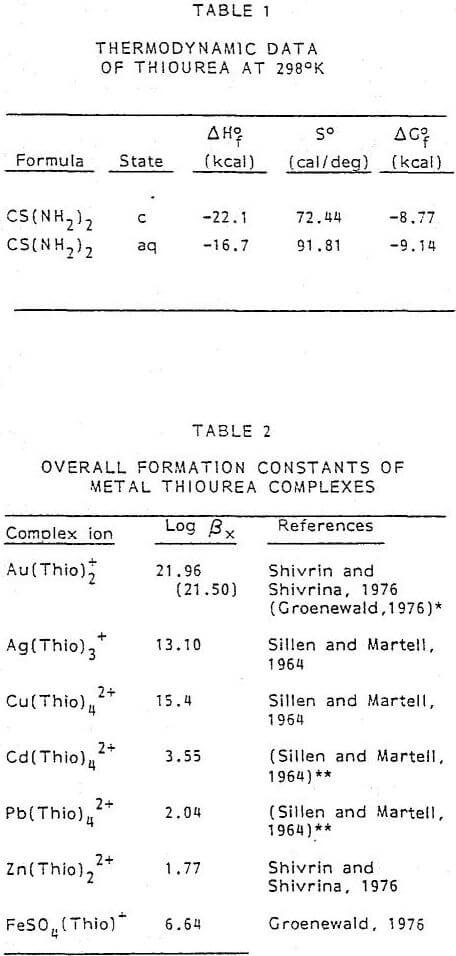
To understand the dissolution chemistry of gold and silver, it is helpful to establish the various equilibria associated with the thiourea system. Unfortunately, there is very little thermodynamic data for aqueous thiourea. The ∆G°f 298 for thiourea was calculated from the limited information available from various sources. The solubility of thiourea as a function of temperature is shown in Figure 1. At 298°K, a saturated solution will contain approximately 142 g/l CS(NH2)2. The heat of solution at 298°K for CS(NH2)2 in water is 5.4 kcal/mole. From this information it was possible to calculate the various thermodynamic properties of aqueous thiourea. Table 1 summarizes the thermodynamic data of thiourea for both the crystal and aqueous form.
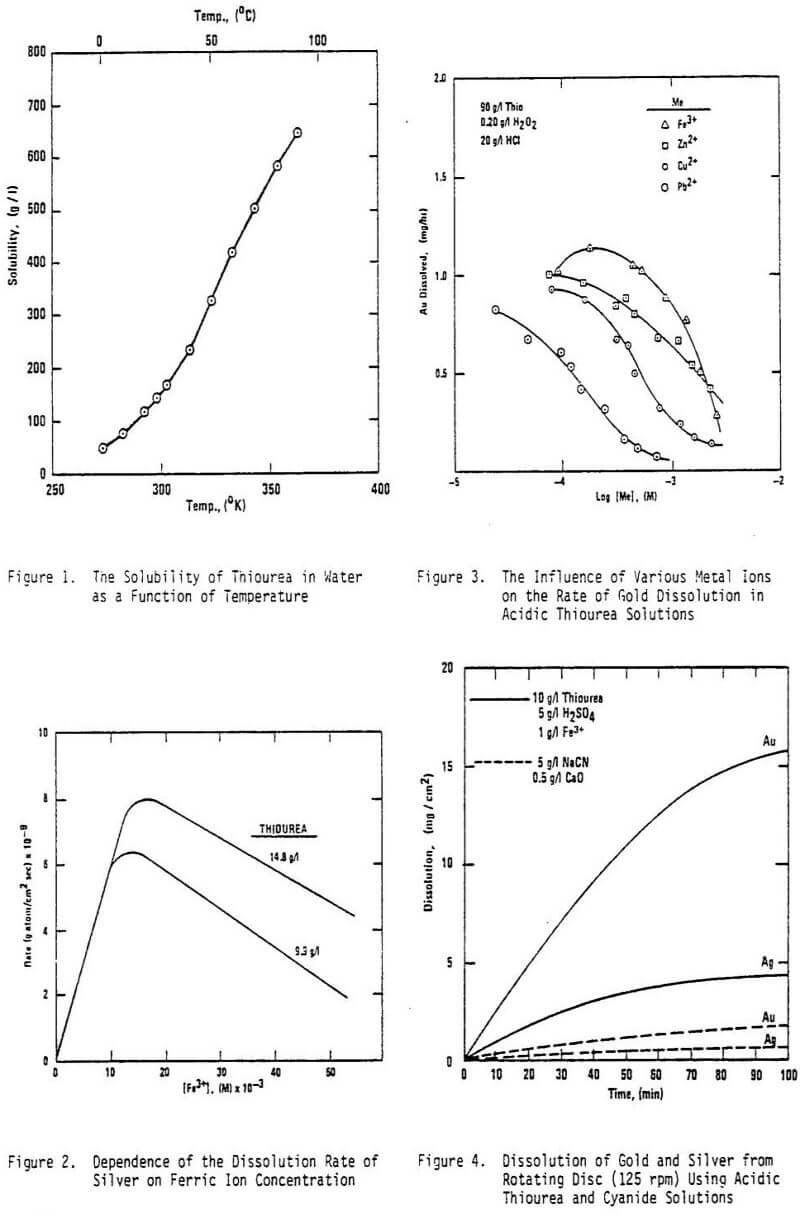
The reactions of thiourea with Au+ and Ag+ are of particular interest; however, reactions with other metal ions assume importance because they have the potential of consuming excessive amounts of thiourea. The nonferrous metals (Pb, Zn, Cd, Cu, etc.) are important in this regard. Furthermore, Fe3+ and Fe2+ thiourea complexes are of consequence in some leaching systems. In a mixed sulfate-thiourea system, the existence of both [FeSO4(CS(NH2)2)] and [Fe(CS(HN2)2SO4 has been established.
Table 2 gives the overall formation constants of a number of metal thiourea complexes. The bis(thiourea)gold(I) ion is the only known thiourea complex of the aurous ion. The predominate thiourea complex of the argentous ion is the tris(thiourea)silver(I) ion, there is some evidence of the existence of the bis(thiourea)silver(I) complex.
There is abundant evidence that in the presence of some oxidants used to dissolve gold and silver, thiourea will undergo oxidation in various stages. The first oxidation product, formamidine disulfide, forms relatively easily in acid solution. Groenewald (1975) has studied the oxidation of thiourea on gold electrodes in acidic solutions. At anodic potentials greater than approximately 0.30 V, thiourea was oxidized to formamidine disulfide. The following redox reaction illustrates the first stage of thiourea oxidation.
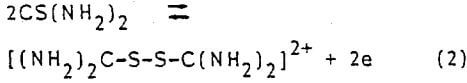
Succeeding reactions proceed at much slower rates, forming oxidation products that include elemental sulfur and even sulfate ions. Some oxidants (e.g., hydrogen peroxide) will oxidize thiourea quite fast at room temperature. Even though the oxidation potential for the Fe3+/Fe2+ couple is sufficiently high to oxidize thiourea, the reaction between ferric ions and thiourea proceeds at a slow rate in sulfate solutions because of the stabilizing effect of the [Fe(SO4) (Thio)]+ complex.
https://www.youtube.com/watch?v=MCMPfrrS_DI
There is considerable proof that formamidine disulfide behaves as an internal oxidizing agent rates observed for 0.84 and 1.40 g/l [Fe3+] were 7.6 x 10-9 and 10 x 10-9 g-atom Ag/cm² sec, respectively. Figure 2 shows the dependence of the rate of silver dissolution on ferric ion concentration at 25°C for solutions containing 9.9 and 19.8 g/l thiourea. Both thiourea levels display two distinct kinetic regions. In the first kinetic region (i.e., low [Fe3+], the rate is controlled by diffusion of ferric ions and increases with increasing [Fe3+]. The second region exhibits a decrease in rate because of a reduction in thiourea concentration (excess Fe3+ consumes thiourea by formation of Fe3+ thiourea complexes). The experimental activation energy was shown to be sensitive to thiourea concentration. At 1.12 g/l [Fe3+], the activation energy was determined to be equal to 9.61, 7.29, and 5.56 k-cal/mole for thiourea concentrations of 4.95, 12.2, and 22.8 g/l, respectively.
Ozone has been proposed as an oxidant for various hydrometallurgical applications. The dissolution of metallic silver in acidic thiourea by ozone was investigated by Chtyan, et al. (1975). Nearly complete (98%) dissolution of -100 mesh Ag was achieved in 90 min using 55.9 g/l thiourea, 68.5 g/l H2SO4 and
0.06 g/l O3.
Although the use of thiourea as a lixiviant for gold and silver was discussed over 90 years ago, just recently renewed interest in this reagent has become apparent. The following general conclusions were found regarding thiourea processes for gold and silver.
- The dissolution of both metallic silver and gold in acidic thiourea medium is much faster than in cyanide solutions.
- Chemical oxidants compatible with acidic thiourea solutions include ferric ion, hydrogen peroxide, ozone, and oxygen.
- The rate of metal dissolution, especially gold, is fastest in the presence of ferric ion. However, ferric ion consumes excessive amounts of thiourea in the formation of iron-thiourea complexes.
- The leaching of refractory forms of gold and silver where an agressive oxidant (e.g., H2O2) is required is viewed impractical because of the rapid oxidation (decomposition) of thiourea.
- The application of thiourea for the treatment of precious metal-containing ores and concentrates is, for many cases, considered uneconomic because of excessive reagent consumption. Thiourea is often consumed by the formation of complexes with impurity metals, and by oxidation to products which are ineffective in complexing gold and silver.
- The selective concentration and purification of gold and silver from thiourea solutions has been demonstrated for a variety of systems, none of which have been developed into a commercial recovery scheme.
- Ion exchange stripping processes involving gold(I)-cyanide and gold(l)- thiourea systems possess distinct possibilities for the processing of gold.
- Electrowinning of gold and silver from thiourea solutions requires a diaphragm cell to separate anodic and cathodic processes. This eliminates sulfur contamination of the cathode and avoids attack of the cathode by oxidants formed at the anode.