Table of Contents
As a part of a research program designed to help minimize national requirements for new mineral commodities by maximizing recovery of metals from domestic secondary resources, the Bureau of Mines investigated the development of economic methods for recovering copper and associated metals from complex electronic scrap. Three types were selected for research:
- multiple-pin plugs and connectors,
- obsolete aircraft radio assemblies, and
- the magnetic fraction of shredded electronic scrap.
The plugs and connectors, after incineration to remove plastic components and melting, yielded a brittle ingot, as did the internal portion of the radio assemblies. The brittle ingots were ground to minus 35-mesh for processing, and the magnetic material was used in the as-received condition.
A process using pretreated or raw scrap as a copper precipitant in various concentrations of acidulated copper sulfate solution was developed as a means of effecting an initial separation and upgrading in the form of high-grade cement copper containing all or most of the precious metals.
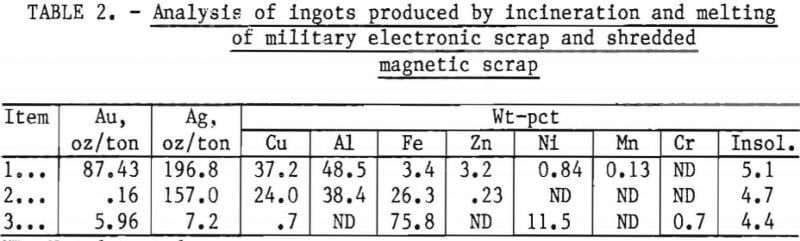
A long-term evaluation program was initiated to determine the content of various types of military electronic scrap. As an outgrowth of this program, DPDS hand-sorted about 3 tons of scrap into high-copper material that was amenable to existing technology, and a fraction, lower in precious-metal and copper content and high in aluminum, for which an analysis is shown as item 1 in table 2. The physical appearance of this material is shown in figure 1.
A second type of refractory scrap was represented (item 2 of table 2) by obsolete radio assemblies, referred to by the disposal agencies as “black
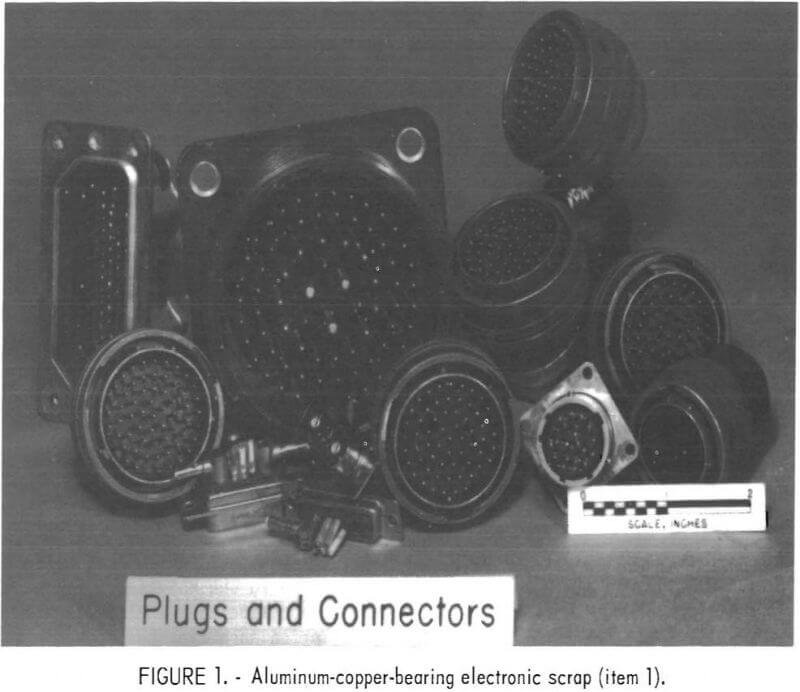
boxes.” A disassembled black box is shown in figure 2. A third type of material (item 3 of table 2) treated in the investigation was furnished by the Bureau’s Avondale Research Center. This material, shown in figure 3, consisted of the magnetic fraction of shredded electronic scrap obtained from Naval Ordnance. This scrap contained predominantly iron and nickel with a small amount of gold and silver.
Metal obtained from incinerating and melting the multiple-pin plugs and connectors, item 1 of table 2, is difficult and expensive to refine because of its high aluminum content. Therefore, this material was chosen as the first type of refractory scrap for this study.
The ingots produced by incinerating and melting the multiple-pin plugs and connectors (item 1 of table 2), and the “black boxes” (item 2 of table 2), are not amenable to separation and purification by conventional treatment. Therefore, the Bureau initiated a study to determine whether the copper and precious metals in refractory alloys could be recovered by a process that consisted of using finely divided scrap as a cementation agent for copper, melting the precious-metal-bearing copper precipitate into an anode, and

electrorefining the anode to produce a pure copper cathode and a precious- metal-bearing sludge that could be treated by conventional methods for the recovery of gold and silver. Because the Avondale magnetic scrap was mostly iron, an excellent cementation agent for copper, this approach would appear to be applicable also to this third type of scrap. This paper describes the results of this research.
Experimental Procedures
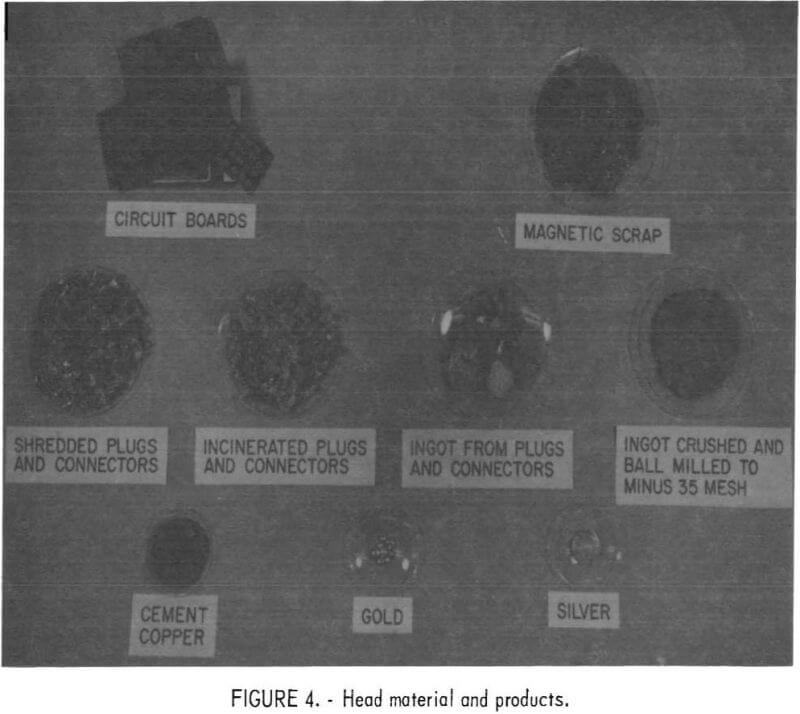
Each of the three types of scrap described above, and shown in figure 4, had to be treated by a different variation of the basic process. Therefore, the treatment used for each type of scrap is discussed separately.
Multiple-Pin Plugs and Connectors
The initial scrap selected for research was the metal obtained by incinerating and melting aluminum-bearing plugs and connectors. The resulting brittle ingots were broken up and roll-crushed to minus 10-mesh, mixed, and sampled. The products obtained from this material are shown in figure 4.
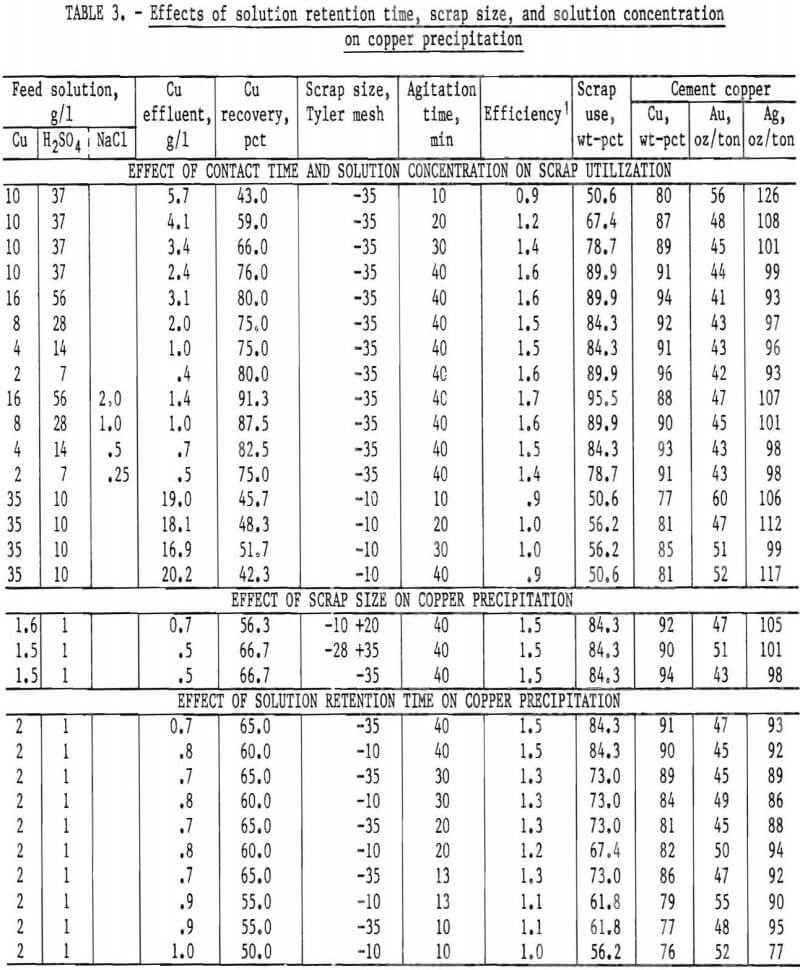
Based on the assays shown in table 2 (item 1), the aluminum, iron, and zinc content of 1 gram of scrap has a potential of precipitating 1.78 grams of copper from a copper sulfate solution. Several series of tests were made to determine the conditions necessary to make maximum use of this potential. A number of preliminary tests were made by agitating a measured amount of solution containing various concentrations of copper and sulfuric acid with a weighed amount of scrap for varying periods of time, followed by filtration and washing the precipitate. In each test an excess of copper-bearing solution was used to insure maximum use of the scrap. Under these conditions it was found that 40 minutes contact time produced a precipitate containing from 88 to 96 pct Cu, with excellent utilization of scrap. The results with minus 35-mesh scrap were consistent over a wide range of copper and sulfuric acid concentrations. A slight increase was noted for copper recovery and scrap utilization with the use of NaCl. The minus 10-mesh scrap was less efficient and produced a lower grade cement copper regardless of contact time. The results are shown in table 3.
 Several tests were made to determine the maximum amount of copper precipitated per gram of various
Several tests were made to determine the maximum amount of copper precipitated per gram of various
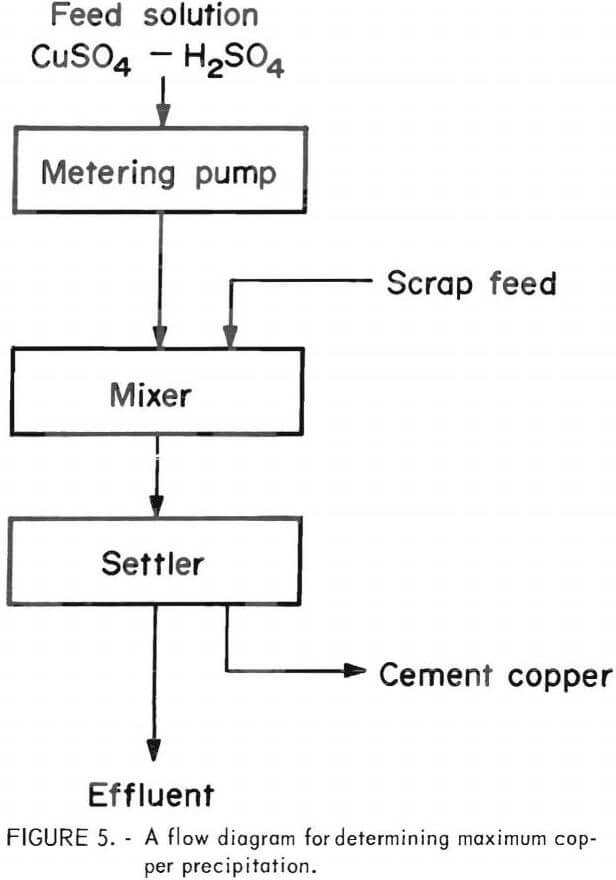
size fractions of scrap, and the grade of the resulting cement copper. The tests were carried out by placing a weighed sample of scrap in a 1-liter mixer fitted with an overflow to a 1-liter settler, followed by pumping an acidulated copper sulfate solution into the system at a definite rate until the effluent showed unprecipitated copper. A flow diagram of the process is given in figure 5. The results, shown in table 3, indicate the best copper recovery was effected by the use of finer scrap. Since the material was difficult to grind to very fine sizes, it was decided that it would not be feasible to use particle sizes finer than minus 35-mesh even though finer particle sizes might improve effectiveness.
A similar set of tests in the same equipment to determine the effect of contact time when using minus 10- and minus 35-mesh scrap with solution concentration remaining constant. The results, given in table 3, show the best efficiency and copper grades were obtained with 40 minutes contact time for both minus 10- and minus 35-mesh scrap. The efficiency of minus 35-mesh material remained relatively constant at 30, 20, and 13 minutes contact time, whereas the efficiency of the minus 10-mesh scrap decreased steadily over the same periods of time.
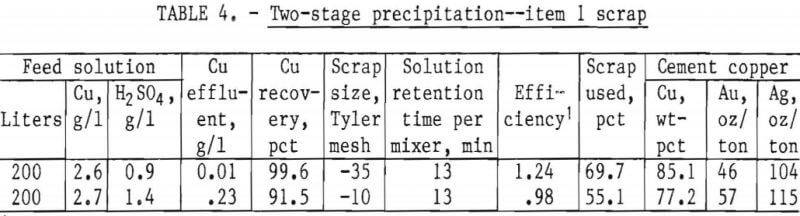
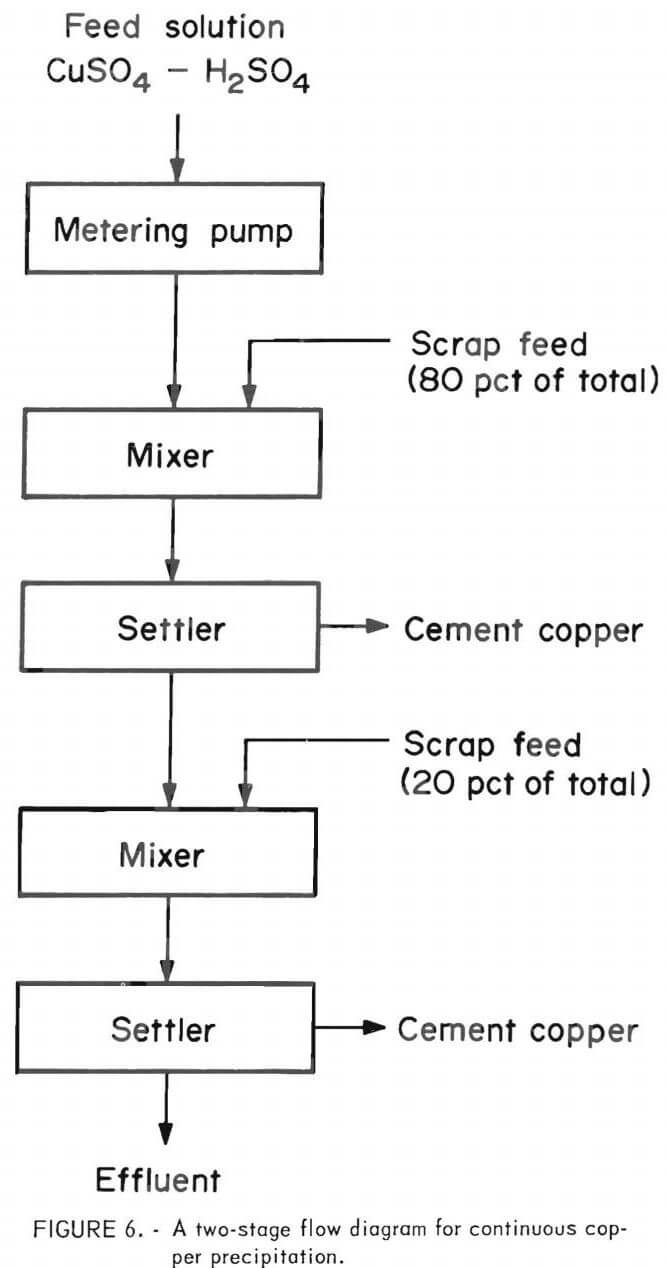
In the interest of obtaining the maximum copper precipitation in a reasonable amount of time, tests were designed for minus 35-mesh and minus 10-mesh scrap using two mixer-settlers in series. The scrap was added hourly at the rate of 1 gram per 1.32 grams of copper available, with 80 pct in mixer 1 and 20 pct in mixer 2. Acidulated copper sulfate solution was pumped into the system at a rate equivalent to about 13 minutes contact time in each vessels Very little reaction was noted in the settlers, even though about 90 pct of the cement copper was collected in these vessels, indicating essentially complete reaction in the mixers. The flow diagram used in these tests is shown in figure 6. The results given in table 4 show that minus 35-mesh scrap, treated in a two-stage system, will produce a precipitate containing in excess of 85 pct copper, a product that is amenable to subsequent refining. A recovery of 99.6 pct of the copper was obtained from the head solution with a utilization of 69.7 pct of the scrap added. A similar test made on minus 10-mesh scrap, also shown in table 4, indicates substantially lower copper recovery and scrap utilization than the minus 35-mesh material.
Obsolete Aircraft Radio Assemblies
A second type of scrap was represented by obsolete aircraft radio assemblies, referred to by the Defense Disposal agency as “black boxes.” It was determined that the outer cover of a “black box” can be removed in about 8 minutes by one person to reclaim over one-third of the original weight as a clean aluminum scrap product. The remaining inner assembly, when shredded, incinerated, and melted, yields a brittle ingot that can be crushed to a fine size for use as a copper precipitant. An analysis of an ingot prepared in this manner is shown as item 2 in table 2.
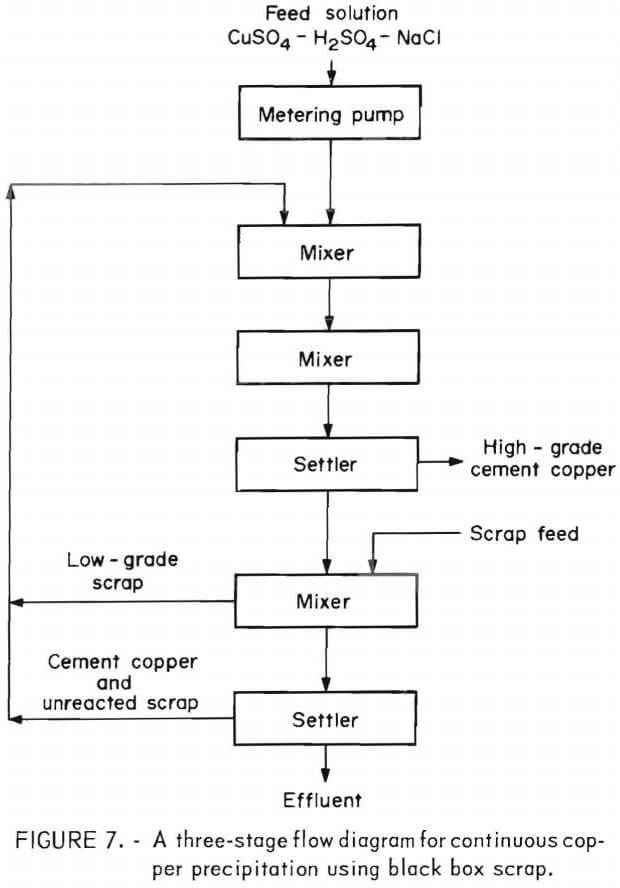 The ingot was crushed and ball milled to minus 35- mesh for use in subsequent testing as a copper precipitant. A number of tests were made, similar to those made on the first type of scrap, but the results were poor until a mechanical change was made in the system and sodium chloride was added to the head solution. The best results were obtained from a system consisting of two 1-liter mixers in series followed by a 1-liter settler, this in turn followed by a mixer and settler of similar size. A large excess of minus 35- mesh scrap was placed in the last mixer for the purpose of insuring a low-grade effluent, and as a source of feed scrap to the first mixer. The amount of scrap advanced per hour from the last mixer to the first mixer was adjusted to be somewhat less than that necessary for complete precipitation of the copper entering the system. A flow diagram of this process is given in figure 7.
The ingot was crushed and ball milled to minus 35- mesh for use in subsequent testing as a copper precipitant. A number of tests were made, similar to those made on the first type of scrap, but the results were poor until a mechanical change was made in the system and sodium chloride was added to the head solution. The best results were obtained from a system consisting of two 1-liter mixers in series followed by a 1-liter settler, this in turn followed by a mixer and settler of similar size. A large excess of minus 35- mesh scrap was placed in the last mixer for the purpose of insuring a low-grade effluent, and as a source of feed scrap to the first mixer. The amount of scrap advanced per hour from the last mixer to the first mixer was adjusted to be somewhat less than that necessary for complete precipitation of the copper entering the system. A flow diagram of this process is given in figure 7.
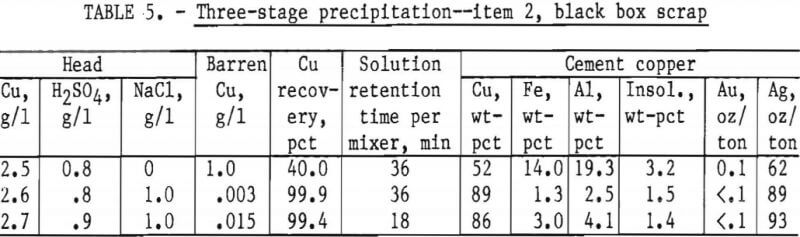
In each test about 20 liters of feed solution was passed through the system, followed by measuring, weighing, and assaying the final products. The precipitates from mixers 1 and 2 and settler 1 were combined as a finished cement copper product. The results, given in table 5, show the advantage in using NaCl in the feed solution. The decrease in retention time from 36 minutes to 18 minutes did not seriously reduce copper recovery or final cement copper grade.
Magnetic Electronic Scrap
The third type of scrap treated was the minus ½- plus ¼-inch magnetic fraction of shredded electronic material submitted by the Bureau’s Avondale Research Center. An analysis of the scrap, designated item 3, is shown in table 2.
Preliminary tests in 1-liter mixer-settlers indicated a utilization of 85 to 90 pct of the scrap as a copper precipitant and produced a precipitate containing in excess of 90 wt-pet copper, over one-half of the precious metals, and a residue of unreacted magnetic scrap enriched in nickel, chromium, cobalt, gold, and silver.
In order to obtain the vigorous scrubbing action needed to maintain the precipitating reaction with this type of scrap it was found necessary to build a tumbler. This was constructed from an 8-inch-diameter clear plastic cylinder 8 inches in length. The cylinder was fitted with lifters and the ends were sealed with ½-inch by 12-inch-diameter plastic disks that served as trunnions. A 3/8-inch hole bored in the inlet end served as a guide for a flexible solution feed tube. A 2-inch-diameter plastic tube, covered with a 20-mesh stainless steel screen, was fitted in the outlet end. Agitation was accomplished by placing the tumbler on a set of variable speed rolls.
A number of experiments showed that the best results were obtained when rotating the tumbler at 42 rpm and with a solution retention time of approximately 20 minutes. In order to make maximum use of the scrap and produce an acceptable barren solution it was found necessary to treat the effluent from the tumbler with minus 35-mesh scrap in a mixer-settler arrangement. A flow diagram of this process is given in figure 8.
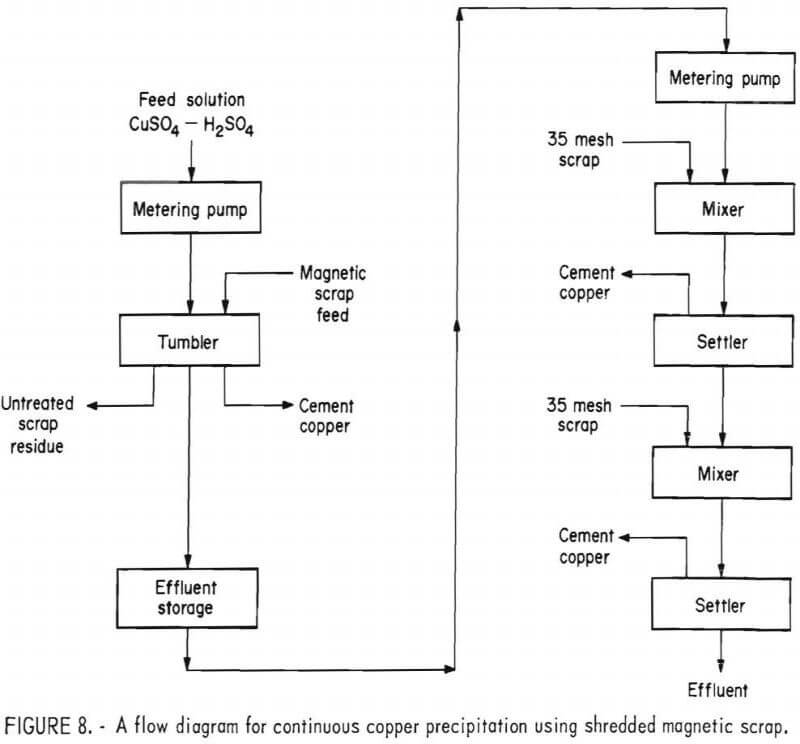
In tests made with this system the tumbler was charged with 2,000 grams of minus ½- plus ¼-inch magnetic scrap and rotated at 42 rpm. An acidulated copper sulfate solution, containing a minimum of 2 g/l H2SO4, was pumped into the tumbler at the rate of 100 ml/min until no further precipitation was noted. During the test, the tumbler effluent was discharged to a surge tank, from which it was pumped into the mixer-settlers at 75 ml/min. The differential in throughput of the two units allowed sufficient time to discharge the residue from the tumbler and reload with fresh scrap, while still continuing solution flow to the mixer-settlers, thus maintaining essentially a continuous system.
In each test the final residue in the tumbler contained several hundred grams of minus 6-mesh pellets. When the unreacted scrap was removed with a magnet, the remaining pellets were found to contain in excess of 99 pct Cu.
The results of one complete 2,000-gram cycle using a total of 320 liters of feed solution containing 5.2 g/l Cu and 4.9 g/l H2SO4 are shown in table 6.
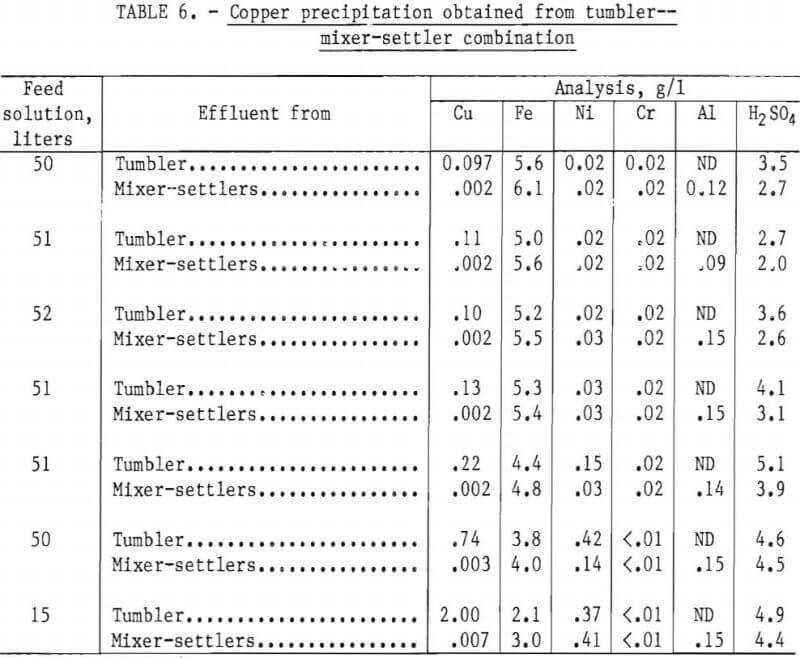
The final barren assays show that 99.96 pct of the available copper in the feed solution was precipitated, and reported in the various products as shown in table 7.
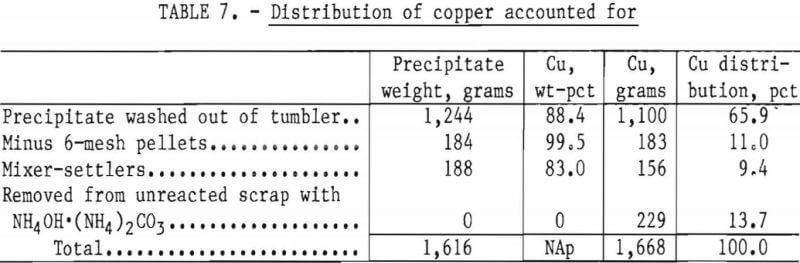
An analysis of the combined precipitate showed it to contain, in weight-percent, 89 Cu, 1.1 Fe, 0.32 Pb, 0.36 Sn, 1.5 insolubles, and, in ounces per ton, 4.0 Au and 11.5 Ag.
The total precipitate shown in table 7 was melted in an induction furnace without flux. An anode, representing 90 pct of the charge weight, was obtained from the melt; the balance, containing most of the impurities, remained as a sinter. The anode was placed in a polypropylene sock and electrorefined in a solution containing 150 g/l H2SO4 and 40 g/l Cu at a current density of 12 amp/ft². These conditions were selected because previous electrorefining tests with similar values had produced a dense, smooth cathode deposit. Assays of the various products are shown in table 8.
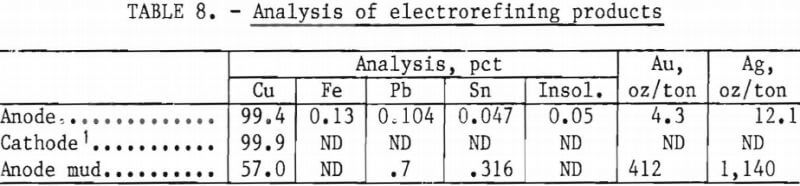
The anode mud can be further refined by standard procedures for the recovery of gold and silver.
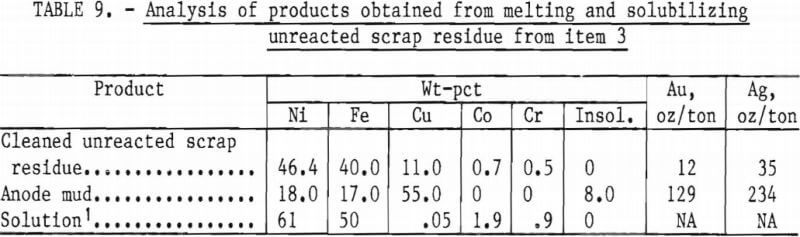
The cleaned unreacted scrap residue, amounting to 11.0 pct of the original charge, was melted in an induction furnace and cast into an anode. An assay of the metal is shown in table 9.
The anode was suspended in an electrolytic cell fitted with a graphite cathode and partially solubilized in an electrolyte containing 150 g/l H2SO4. The cell was operated 145 hours at 0.5 volt and 1 ampere, resulting in the solubilization of 63 grams of the anode. The solution values, shown in table 9, might be treated by presently used procedures to recover the Ni, Co, and Cr in commercial form. The anode mud, see table 9, can be treated by standard procedures for the recovery of the precious metals.
Conclusions
Electronic scrap generated from multiple-pin connectors and the inner assemblies from “black boxes” can be melted to produce a brittle metal that is easily crushed and ball milled to a fine size. This material, when agitated with an acidulated copper sulfate solution in a countercurrent system, will produce a precipitate containing 90 to 95 pct Cu and all of the associated precious metals. The cement copper thus obtained can be beneficiated by fire refining to produce high-grade anode material for subsequent electrorefining to cathode copper and an anode mud from which precious metals can be recovered.
The magnetic fraction of shredded electronic scrap can be used as a cementation agent to produce cement copper exceeding 90 pet in purity when agitated in an acidulated copper sulfate solution. About 90 pct of the scrap is consumed in the copper cementation reaction producing a cement copper containing over one-half of the original precious metals, and leaving a residue enriched in nickel, chromium, cobalt, gold, and silver. The cement copper can be processed in the same manner as that already described for the other types of scrap, and the residue, cast into an anode, can be dissolved electrolytically to produce a solution containing a relatively high concentration of nickel and cobalt that might be processed to recover these metals, and an anode mud containing copper, gold, and silver that can be reprocessed by conventional techniques to recover these metals.
