The mission of the Bureau of Mines minerals research program is to help improve minerals processing technology so that the Nation’s mineral needs can be fulfilled in a manner that reduces waste and ensures that minerals and metals are processed, used, and recycled at acceptable economic, social, and environmental costs. In accordance with this mission, a research program was conducted to investigate a new direct flotation method for the recovery of potash without prior removal of insoluble slimes. Methods were devised that are effective on high- insoluble-slime-bearing carnallite and sylvinite ores.
The Permian Basin near Carlsbad, N. M., has been a source of high-grade sylvinite ores during the last 40 years. As these high-grade sylvinite ores are depleted, low-grade sylvinite ore, containing abundant insoluble slimes, and carnallite ores are becoming the future sources for potash production. These ores contain 1% to 8% water insoluble slimes which must be removed prior to potash flotation because of their high adsorptive capacity for the amine collectors used in potash flotation. These insoluble slimes conventionally are removed by scrubbing the ore, followed by hydroclassification to separate the slimes from the coarse ore, as reported by G. E. Atwood (1953) and J. S. Browning (1972). Residual insoluble slimes not removed by hydroclassification are blinded with reagents such as guar gum or starch to prevent interference in subsequent potash flotation.
Another method that is gaining in commercial usage is insoluble slimes flotation prior to potash flotation [W. B. Brogiotti (1974), B. S. Fee (1973), P. Thompson (1979), C. F. William (1960), and V. A. Zikov (1975)]. In these processes, the slimes are flocculated and then floated with a variety of reagents. Losses of potash to the slimes product are generally less than in the mechanical desliming process.
Because of the significant potash losses in the discarded insoluble slimes and the difficulty in removing insoluble slimes, improved methods are needed that will minimize these problems. In this regard, a direct flotation method has been investigated as an alternative to mechanical desliming and as an improvement on flotation desliming.
Experimental Materials
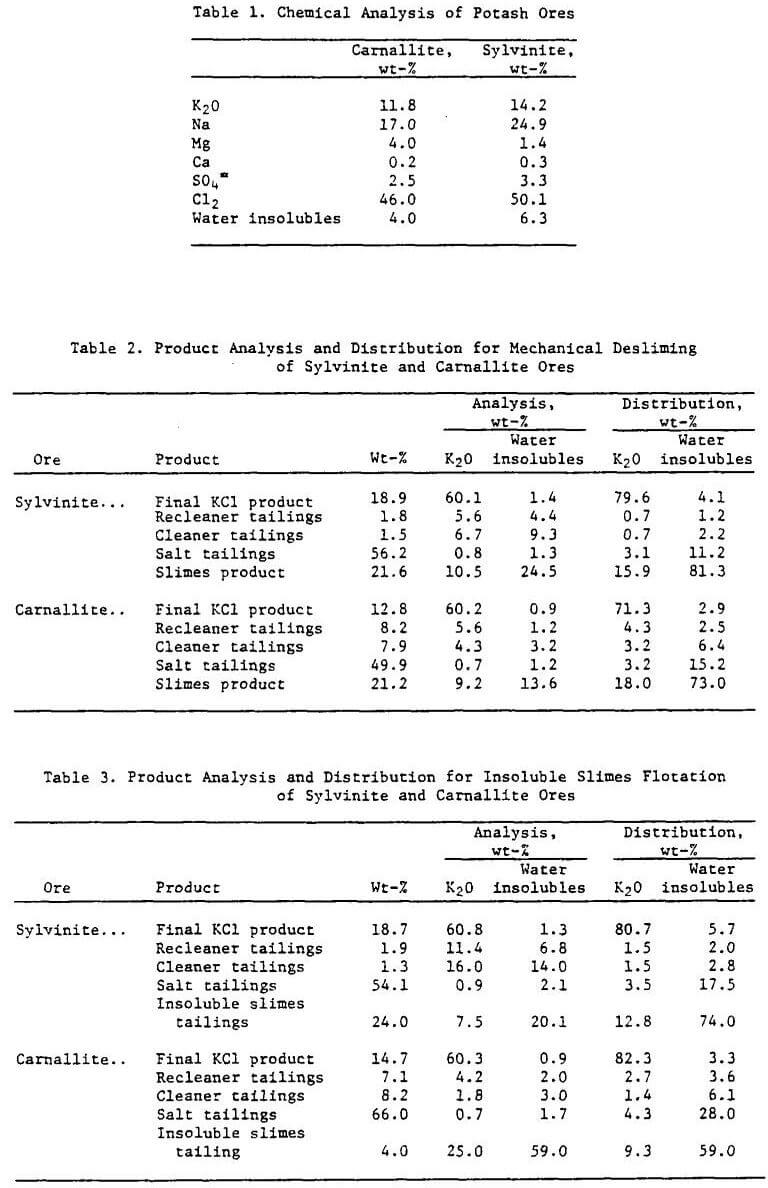
High-grade carnallite and low-grade sylvinite ores containing high amounts of water insolubles from the Carlsbad, N. M., area were used in this study. Chemical analyses of the ores are shown in Table 1.
Petrographic and X-ray diffraction analyses of the carnallite ore indicated that it contained, in weight-percent, 43.0 carnallite, 7.2 sylvite (KCl), 43.2 halite (NaCl), and 4.0 water-insoluble slimes. Some carnallite and sylvite contained minor amounts of occluded hematite, which gave the minerals a distinct red color. Minor amounts of polyhalite (MgSO4·K2SO4·2H2O), kainite (KCl·MgSO4·3H2O), and leonite (MgSO4·K2SO4·4H2O) were also present. The water-insoluble fraction of the ore contained abundant magnesite, chlorite, and illite.
Petrographic and X-ray diffraction analyses of the sylvinite ore indicated that it contained, in weight-percent, 22.5 sylvite, 64.3 halite, and 6.3 water-insoluble slimes. The sylvite had a distinct red color due to hematite coloring. Minor amounts of polyhalite, kainite, and leonite were also present. The water-insoluble fraction of the sylvinite ore contained magnesite, chlorite, and illite, as did the carnallite ore.
Minus 0.10-m (4-inch) carnallite and minus 0.02-m (¾-inch) sylvinite ores were prepared for bench-scale flotation by stage-crushing and grinding through 1.70 mm (10 mesh) using a jaw crusher and roll crusher and then splitting the crushed ore into 1-kg (2.2-lb) charges. Stage crushing was used to avoid producing excessive fines, which report to the slimes product and reduce the overall potash recovery. Although the screen analysis varied slightly for each ore, no more than 18% of the final crushed weight was finer than 150 µm (100 mesh).
A synthetic equilibrium brine containing, in weight-percent, 1.6 K, 0.8 Na, and 6.5 Mg was used for the carnallite flotation work. The density of the brine was 1.278 g/cm³. A brine obtained from a potash plant near Carlsbad, N. M., containing, in weight-percent, 4.5 K and 5.8 Na was used in flotation of the sylvinite ore. This brine density was 1.254 g/cm³.
Potash flotation reagents used in this study are all commercially available and include Armeen TD¹ (a primary alphatic tallow amine) neutralized with HCl, Barrett’s 634 flotation oil emulsified in tap water, and MRL-201 guar gum insoluble slime blinder.
The insoluble slimes flocculant used was a high-molecular-weight, nonionic polyacrylamide, and the insoluble slimes flotation collector was a cationic surfactant which is a mixture of octadecyl amine and octadecyl guanidine salts of octadecyl carbamic acid reacted with ethylene oxide. Hexanol frother was used as needed in all tests. All reagent consumptions are expressed as kilograms of reagent per metric ton of ore (kg/ton) and pounds of reagent per short ton of ore (lb/ton).
Experimental Procedures
Two-Stage Mechanical Desliming
One-kilogram (2.2-lb) samples of ore were scrubbed with saturated equilibrium brine at a pulp density of 27% solids for 15 min in a Fagergren laboratory flotation cell using a peripheral impeller speed of 4.22 m/s (830 ft/min). The slurry was settled for 1 min, and the suspended solids were decanted onto a vibrating 150-mesh screen. The undersize material was discarded. The oversize material was washed back into the deslimed sample, and the procedure was repeated. The final deslimed material was used as potash flotation feed. This procedure was adopted to simulate commercial operating conditions. Following mechanical desliming and decomposition leach of the carnallite ore, potash flotation was performed.
Decomposition-Leach of the Carnallite Ore
Since carnallite must be decomposed to KCl in order to produce a feed material for potash flotation, various decomposition-leach procedures were tested on a laboratory scale, which led to the adoption of the following standard procedure. The carnallite ore, either as received or deslimed, was diluted to 60% solids pulp density in the equilibrium brine, and the slurry was agitated at a peripheral impeller speed of 3.65 m/s (720 ft/min). Enough fresh water was added to dissolve the MgCl2, leaving a solid KCl product. Approximately 200 kg of fresh water per metric ton of 43% carnallite ore (400 lb/ton) was added dropwise over a 3-hour period, followed by a 1-hour equilibrium period. This procedure produced a stable KCl surface with minimum potassium dissolution. Approximately 9% of the potassium in the carnallite was dissolved in the brine during the decomposition leach. A portion of this potash could be recovered by solar evaporation of excess plant brine.
Insoluble Slimes Flotation
Batch insoluble slimes flotation tests were conducted in a Fagergren laboratory flotation cell. In each test, 1 kg (2.2 lb) of ore was scrubbed for 5 min at a pulp density of 27% solids in a saturated brine at a 4.22-m/s (830-ft/min) peripheral impeller speed to disperse the insoluble slimes. The impeller speed was reduced to 3.65 m/s (720 ft/min), flocculant and collector were added, air was introduced immediately, and an insoluble slimes froth product was collected for 2 min. The respective flocculant and collector additions were 0.1 and 0.2 kg/ton (0.2 and 0.4 lb/ton) for the sylvinite and carnallite ores. The flotation procedure was repeated once on high-insoluble- slime-bearing carnallite ores. All tests were performed at a natural brine pH of 7.0 with total flotation time of 2 to 4 min.
Potash Flotation
The deslimed pulp was diluted to 23% solids pulp density in a Fagergren laboratory flotation cell. The pulp was conditioned for 2 min with MRL-201 insoluble slimes blinder and for an additional 2 min with amine and flotation oil. The respective blinder and collector additions were 0.1 and 0.075 kg/ton (0.2 and 0.15 lb/ton) for the sylvinite ore and 0.1 and 0.1 kg/ton (0.2 and 0.2 lb/ton) for the carnallite ore. After conditioning, a drop of frother corresponding to 0.01 kg/ton (0.02 lb/ton) was added, and a potash flotation rougher concentrate was collected for 1.5 min at a peripheral impeller speed of 4.72 m/s (930 ft/min). This procedure was repeated three times with the carnallite ore and once with the sylvinite ore at reduced flotation reagent additions. The extended number of flotation steps for the carnallite ore is due to the viscosity of the high-magnesium brine, which slows flotation response. The potash rougher concentrates were combined in a 500-g Galigher Model LA500 flotation cell to provide enough material for cleaner flotation. The combined pulp was agitated for 1 min without additional reagents. Air was introduced, and a cleaner product was collected for 2 min. The cleaner concentrate was then placed in the 500-g (1.1-lb) Galigher flotation cell, and a recleaner product was collected for 2 min without additional reagents.
Direct Potash Flotation
Both the carnallite ore (after decomposition leach) and the untreated sylvinite ore were diluted to 23% solids pulp density in a Fagergren laboratory flotation cell. The pulp was conditioned for 2 min with a high-molecular-weight, nonionic flocculant and MRL-201 insoluble slime blinder. The pulp was then conditioned an additional 2 min with amine and flotation oil. The respective flocculant, blinder, and collector additions were 0.15, 0.7, and 0.35 kg/ton (0.3, 1.4, and 0.7 lb/ton) for the sylvinite ore and 0.15, 0.8, and 0.4 kg/ton (0.3, 1.6, and 0.8 lb/ ton) for the carnallite ore. Air was introduced, and a potash rougher concentrate was collected for 2 min. This procedure was repeated three times each for both ores tested. The potash rougher concentrate was then cleaned and recleaned, with no reagents added, to produce the final potash product.
Final Product Leaching
The final KCl concentrates produced by each method contained small amounts of NaCl, which had to be removed to produce a marketable potash product. These products were subjected to a spray water leach at an optimum solid-to-water ratio of 4:1. This removed the fine NaCl while keeping potassium losses at less than 0.5 wt-% of the final product. A product containing approximately 60% K2O was produced by this technique.
Experimental Results and Discussion
Mechanical Desliming
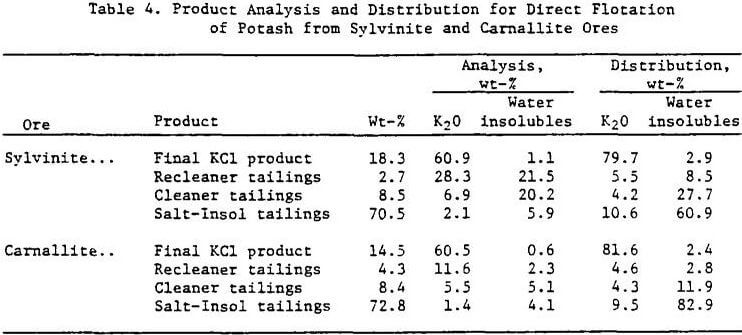
A series of batch tests was performed to evaluate two-stage mechanical desliming. Tests were run on both sylvinite and carnallite ore to determine potassium losses to the slimes product and subsequent potash recovery. Optimum results for the test series are shown in Table 2. The flowsheet shown in Figure 1 was derived from the batch flotation tests.
Sixteen percent of the potassium in the sylvinite ore and 18.0% of the potassium from the carnallite ore reported to the slimes product. Mechanical desliming has three disadvantages: (1) potassium losses to the deslimed products are high, (2) brine requirements are high, and (3) slimes treatment is necessary.
Insoluble Slimes Flotation
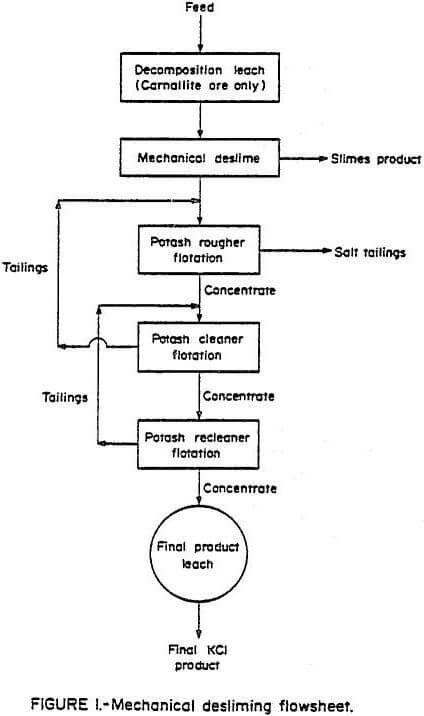
A series of bench-scale flotation tests was performed on both sylvinite and carnallite ore using insoluble slimes flotation to deslime the ore. A flowsheet, derived from batch flotation tests, for this scheme is shown in Figure 2. The same test procedure was used on both ores except that a decomposition leach was performed on the carnallite ore prior to flotation. The optimum results of these tests are shown in Table 3.
Insoluble slimes flotation was shown to produce a slimes product that contained less potassium than the mechanical desliming procedure. The discard products for the insoluble slimes flotation procedure, salt tailings and insoluble slimes, contained considerably more insoluble slimes than the discard from the mechanical desliming procedure. Potassium recoveries were all fairly consistent. The potash recovery from the carnallite ore represents feed to flotation and does not take into account the 9.0% potassium lost to the brine during the decomposition leach stage. Part of this potash might be concentrated by solar evaporation followed by physical beneficiation.
Direct Flotation
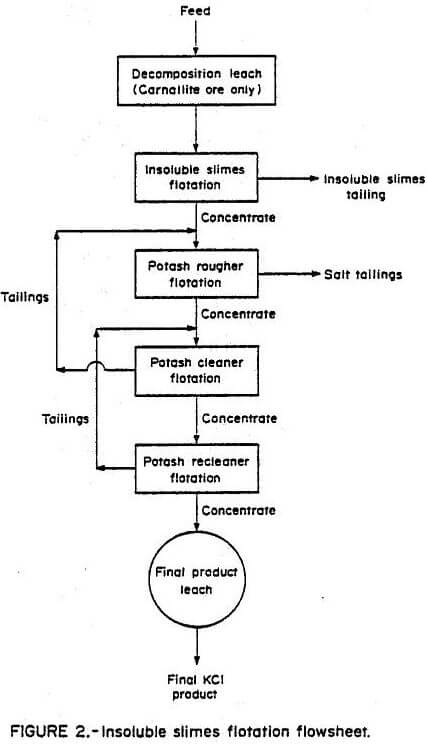
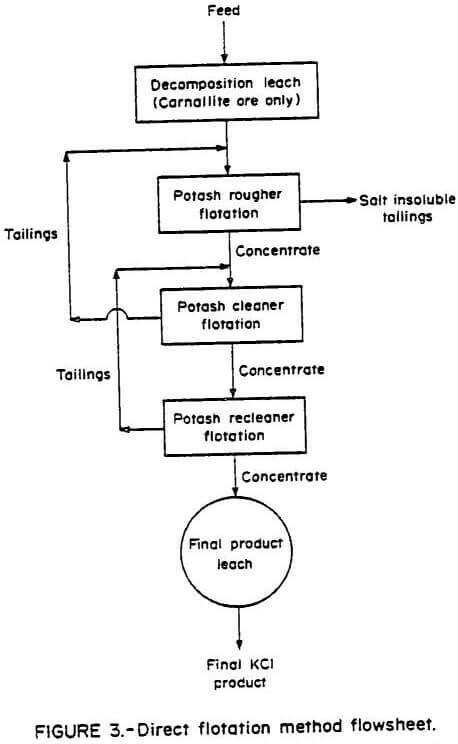
The same ores were subjected to a series of tests using the direct flotation procedure developed by the Bureau of Mines. The flowsheet shown in Figure 3 was derived from batch flotation tests. This method eliminated the necessity of removing the insoluble slimes prior to potash flotation. A decomposition leach was performed on the carnallite ore. The optimum results obtained when using this procedure are shown in Table 4.
The direct flotation method showed a substantial decrease in the amount of potassium discarded with the salt tails and insoluble slimes products. Potash recoveries remained equivalent to those of the other methods investigated. The advantages of the direct flotation method over mechanical desliming and insoluble slimes flotation desliming methods are (1) removal of the insoluble slimes prior to flotation of the potash is not necessary, (2) the detrimental effect of the presence of insoluble slimes on potash flotation is minimized, (3) equivalent or improved removal of insoluble slimes can be achieved, and (4) potassium losses to the discarded insoluble slimes are reduced.
Summary
Laboratory investigations showed that the direct flotation method developed by the Bureau of Mines was effective for recovering potash from high-insoluble-slime-bearing carnallite and sylvinite ores. The technique eliminated the necessity of removing the water-insoluble slimes by either mechanical or flotation desliming. In comparative tests with conventional techniques, the direct flotation method yielded equivalent or improved flotation results with equal or decreased reagent consumption. The direct flotation method recovered 81.6% of the solid phase KCl from carnallite ore and 79.7% of the KCl from sylvinite ore in final products containing 60.5% and 60.9% K2O, respectively.