Table of Contents
As part of the goal to develop technology that can meet critical and strategic needs of minerals and metals, the Bureau of Mines is investigating a procedure for selectively extracting nickel, cobalt, and copper from low-grade domestic laterites. Although the Nation produces less than 10 pct of the primary nickel it consumes and essentially none of its cobalt, extensive resources exist in southern Oregon and northern California containing 0.5 to 1.2 pct Ni, 0.06 to 0.26 pct Co, and about 0.01 to 0.05 pct Cu. These resources are amenable to processing by the Bureau of Mines procedure, for which the results of initial studies regarding characterization of the deposits and development of preliminary process parameters and conditions have been reported. Production would help offset increasing dependence for these strategic and critical metals on foreign suppliers.
An engineering feasibility study and cost evaluation of a commercial size plant has been completed. More extensive pilot plant work is being done. The procedure includes
- Reduction roasting of ground, dry, pelletized laterite with carbon monoxide in a multiple-hearth furnace at about 600° C.
- Leaching the product in ammoniacal-ammonium sulfate liquor with oxygen to extract metal values as soluble amine complexes.
- Solid-liquid separation followed by precipitation of magnesium and manganese with monoammonium phosphate.
- Partial removal of ammonia from the clarified leach liquor.
- Coextraction of nickel and copper with LIX 64N in Kermac 470B in staged mixer-settler operations.
- Reduction of Co III in the raffinate to Co II in a cobalt shot column.
- Extraction of cobalt with the new General Mills reagent LIX 51 in Kermac 470B in mixer-settlers.
- Stripping or selective stripping of the loaded organics of metal values by their respective spent electrolytes for recovery by electrowinning.
The solvent extraction-electrowinning steps for nickel incorporated into the Bureau procedure were adapted from the innovative technology of the SEC Corp. of El Paso, Tex., which separated and recovered nickel from a copper refinery waste product. Separating was possible because copper is specifically extracted by the solvent extraction reagent under acid conditions while nickel is sequentially extracted under basic ammoniacal conditions. The coupling of electrowinning with solvent extraction for metal recovery provided solutions of sufficient acidity from the cells to effect reversal of extraction upon contact in mixer-settlers, a process known as stripping of the organic material. The electrowinning-stripping process was continuous because the small fraction deposited as the solution passed through the cell resulted in acid formation that allowed further stripping. The nickel was electrowon from nickel sulfate electrolyte in a nondiaphragm cell, with internal recirculation, at 60 pct cathode current efficiency. Efficiency was low because of the simultaneous reduction of hydrogen ions that accumulated in the cell in a concentration proportionate to the nickel removal. Accumulation was controlled by depositing or extracting only 3.2 g/l Ni per pass through the cell, the electrolyte then being recycled back through stripping.
The preliminary laboratory-scale nickel electrowinning tests described in this report were conducted parallel to the laterite investigations to determine the effect of operating variables in this procedure on current efficiency and energy consumption so as to enable the selection of optimum parameters, to compare results to those obtained with a diaphragm cell, and to apply the parameters where applicable to laterite processing.
Cobalt hydrometallurgy and electrowinning from various domestic ores was studied by the Bureau’s Boulder City, Nev., Experiment Station during World War II and up until 1948. Electrowinning procedures described in the final report included the use of pure cobalt sulfate electrolyte in nondiaphragm cells in the method attributed to Roentgen and Giesen. A short pilot plant investigation showed favorable results in runs of up to 48 hours. The use of similar cobalt sulfate electrolyte has since been applied industrially in Katanga, Zaire. Other research has shown that optimum conditions for depositing cobalt from sulfate electrolyte require limiting extraction to only 1.2 g/l Co and include 50 g/l Co electrolyte feed with approximately 2 g/l H2SO4, 20 Na2SO4, and a temperature of 50° to 60° C, which permits current efficiency of 90 pct and an energy requirement of 1.5 kwhr/lb Co. The method of Roentgen and Giesen with a nondiaphragm cell and pure C0SO4 electrolyte was adapted to the Bureau procedure for low-grade laterites because of its compatibility with solvent extraction.
Experimental Equipment and Procedure
Most of the electrowinning tests were conducted in a 4.5-liter acrylic cell, 15 x 20 x 16.5 cm, with a single cathode, 15 x 16 cm, of either titanium or stainless steel and two anodes of lead containing 0.05 pct Ca. The electrode spacing was 5 cm. Electrolyte was pumped to and from the cell by a chemical metering pump, while a small fraction of the metal ion content was deposited. The pregnant feed was stored in a 28-liter tank and was preheated to 50° to 85° C. Electrolyte was circulated within the cell by an external centrifugal pump, which had intakes located under the anodes and outlets spaced along tubing running under and parallel to the single cathode. The outlets were 0.5-mm-diam holes drilled alternately at 22.5° from either side of the top center of the tubing at a spacing of 1.25 cm to provide upward jetting at a flow rate of 1.1 l/m. (See fig. 1.) Evaporative losses and misting were minimized by floating polypropylene spheres, which covered the exposed electrolyte surface.
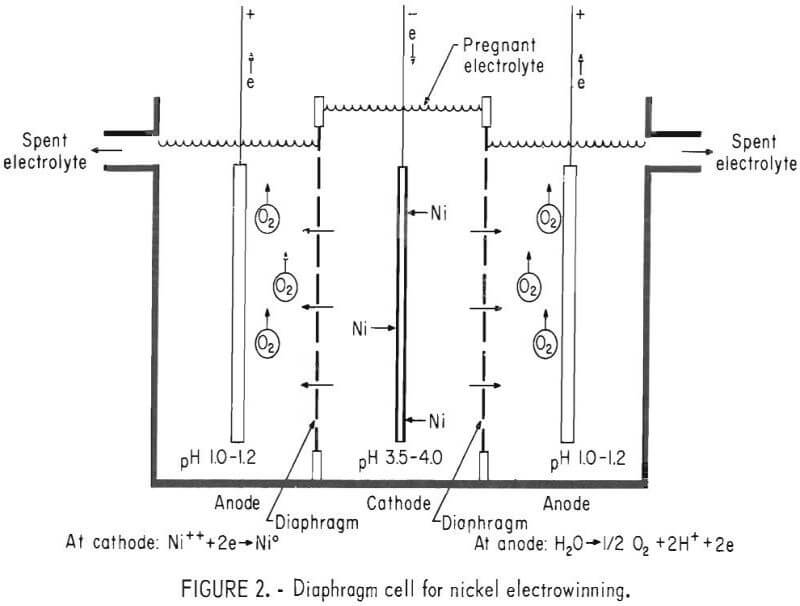
A diaphragm cell was also constructed that was without internal recirculation and was longer, 25 cm, to provide room for diaphragms between the electrodes, which were spaced at 9.5 cm. The diaphragms were of Terylene, a tightly woven P & S Textiles, Inc., synthetic fiber cloth, quality No. 488 or No. 1666. The diaphragms were each a single separating sheet of cloth supported by acrylic frames bonded and sealed along the edges of the cell with silicone rubber sealant. Electrolyte introduced to the catholyte compartment flowed through the cloth at about 20-mm head pressure to the anolyte, where it was pumped from overflow channels to surge tanks. (See fig. 2.)
Auxiliary heating was obtained with a quartz-enclosed resistance heater placed in the cell or recirculating line. The regulated cell current was monitored by a digital ampere-minutes meter. The voltage drop between electrodes was measured at the electrodes, so energy consumption is for electrowinning only. Anodes were removed from the cell between tests for rinsing and storing in a dry rack. Synthetic electrolytes were prepared by dissolving reagent grade NiSO4 or CoSO4 in distilled water and adjusting the pH to 3.5 with H2SO4.
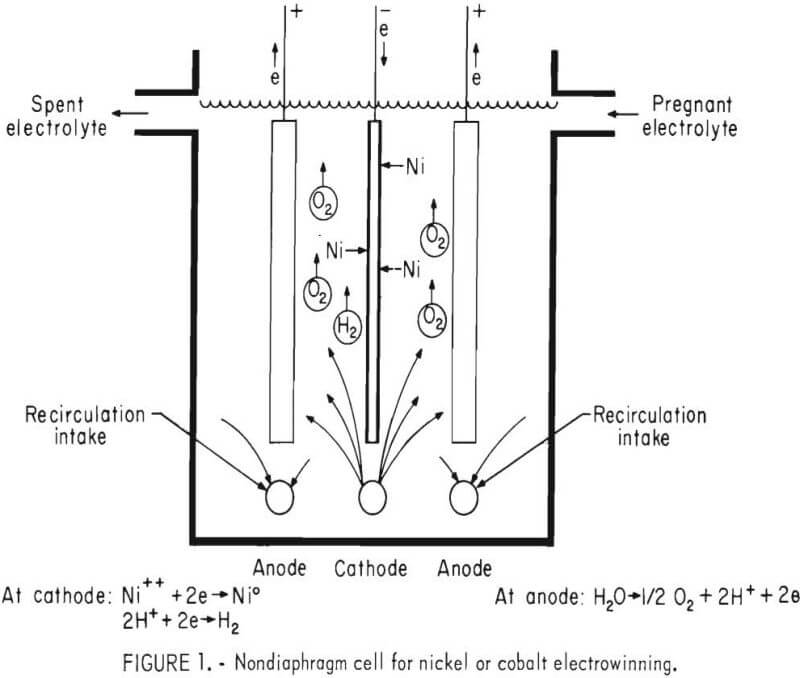
During electrolysis, metal ions that are deposited result in the generation of H2SO4; therefore, experimental electrolytes that had passed through the cells were regenerated for future use by reacting them with basic metal carbonate to neutralize the acid and replace the metal ions. The amount added was determined by titration of the acid with standardized base. Residual dissolved CO2 was removed by boiling and recooling the solutions, after which the pH was again adjusted to 3.5. Later, the concentration of metal ions was adjusted to the desired level following determination of concentration by colorimetric analysis.
The effects of four variables on electrowinning nickel were examined: (1) nickel concentration, (2) nickel extraction, (3) current density, and (4) temperature. Each of these was tested at different levels, including levels constituting the general parameters of the SEC Corp.’s operation. Emphasis was placed on energy consumption, to which results were compared, tabulated, and plotted. Each test was run for about 3,000 amp-min, generally about 5 hours, and produced flat sheets of ductile metal. Later the effects of sodium sulfate and boric acid additions were examined.
The initial conditions for nickel electrowinning were (1) a cell-feed electrolyte of 90 g/l Ni with nickel present as NiSO4 , pH 3.5, (2) a temperature of 50° C, (3) a current density of about 2.15 amp/dm² (20 amp/ft²), and (4) a metal extraction, or nickel drop per pass, of 3.2 g/l.
Initial cobalt electrowinning was conducted using parameters suggested by earlier Bureau of Mines researchers such as a concentration of 40 g/l Co in CoSO4 electrolyte at 60° C.
 Experimental Results and Discussions
Experimental Results and Discussions
Effect of Nickel Concentration
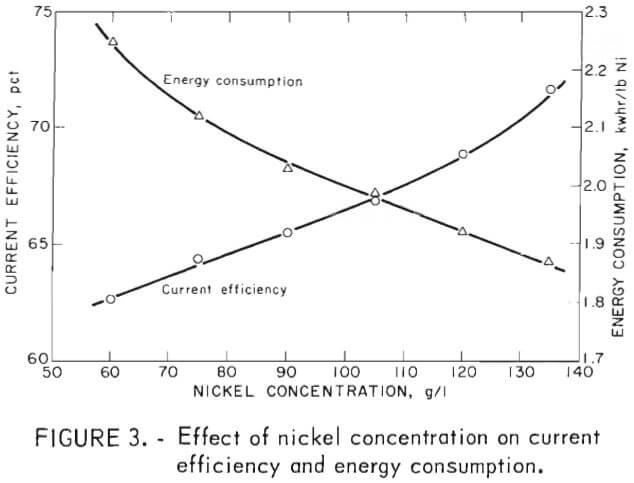
The effect of nickel concentration in the electrolyte on current efficiency and energy consumption was determined by testing with electrolytes containing between 60 and 135 g/l Ni. Other variables were held constant at the initial levels described above. Figure 3 shows that current efficiency increased from 60 to 72 pct, while energy consumption decreased from 2.25 to 1.86 kwhr/lb Ni, as nickel concentration was increased. Because the electrical energy consumption rate was continuing to decline as nickel concentration increased, the data suggest that concentration should preferably be as high as is sensibly practicable depending on concentration-related problems that might arise, such as crystal formation or entrainment of organic phase during stripping.
Effect of Nickel Extraction and Hydrogen Ion Concentration
The effect of nickel removal by electrodeposition is inherently associated with the hydrogen ion concentration because of the electrochemical reactions which take place, as follows:
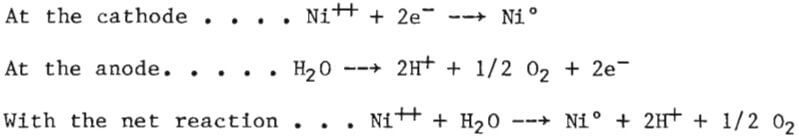
The electrode potential required for decomposing sulfate ions is much greater than for decomposing water; therefore, the sulfate decomposition reaction does not proceed. The hydrogen ions generated by the anode reaction are more easily reduced at the cathode than those of nickel and, in spite of their comparatively low concentration, compete readily for discharge, as follows:

This reaction is simply electrolysis water and is the cause of loss in current efficiency for depositing nickel. As can be seen by the disappearance of hydrogen ions in the net reaction, the reaction does not alter the hydrogen ion concentration, which is dependent on the nickel deposition. The ratio of current reducing hydrogen to that reducing nickel is linearly related to the relative gram equivalent concentration of the hydrogen ions. The strength of these ions in the electrolyte, or the acidity, is equal to the gram equivalent of the nickel deposit divided by the volume of electrolyte, plus the initial concentration, and can be determined by calculation or, preferably, simply by titration.
Current efficiency was found to vary inversely with extraction, or nickel drop, because of the concomitant increase in hydrogen ion. Extractions of 2.37, 3.30, and 4.53 g/l Ni were taken in three tests after the cell was equilibrated at a given constant flow rate of electrolyte. Current efficiency decreased from 74 to 59 pct over this range. The amount of electrical energy required for deposition increased rapidly from 1.82 to 2.24 kwhr/lb Ni. (See fig. 4.)
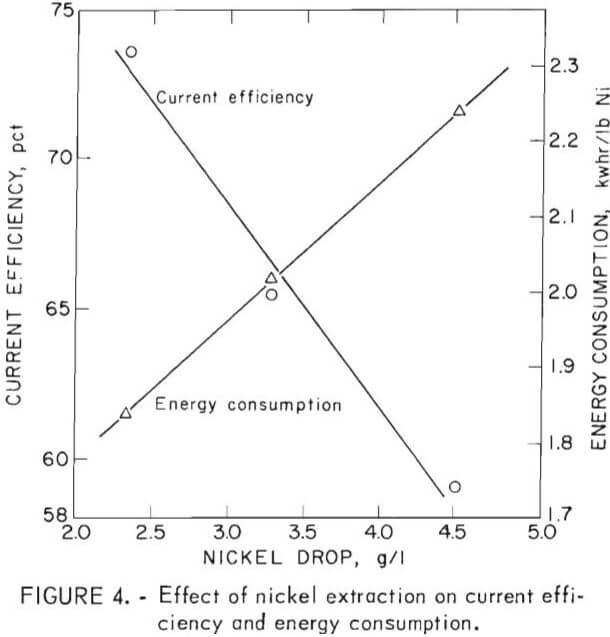 Energy costs therefore rise quickly for increasing nickel extraction. On the other hand, decreasing the extraction requires the transport of rapidly increasing amounts of electrolyte between the electrowinning cells and solvent extraction stripping stages, and this results in decreasing efficiency in stripping. Optimization, therefore, constitutes finding a cost-effective compromise between these two interdependent operations.
Energy costs therefore rise quickly for increasing nickel extraction. On the other hand, decreasing the extraction requires the transport of rapidly increasing amounts of electrolyte between the electrowinning cells and solvent extraction stripping stages, and this results in decreasing efficiency in stripping. Optimization, therefore, constitutes finding a cost-effective compromise between these two interdependent operations.
Effect of Current Density
The effect of increasing the cathode current density from 15 to 45 amp/ft² was tested at two extraction levels, 3.2 and 5.1 g/l, while again holding other variables constant at the initial conditions—with the exception, of course, of providing a corresponding flow rate of electrolyte. While only a slight increase in current efficiency was obtained—2.6 and 3.3 pct, respectively—at these two extraction levels, energy consumption increased 51 and 46 pct because of the increase in cell voltage from 3.2 to 4.8 volts. (See fig. 5.)
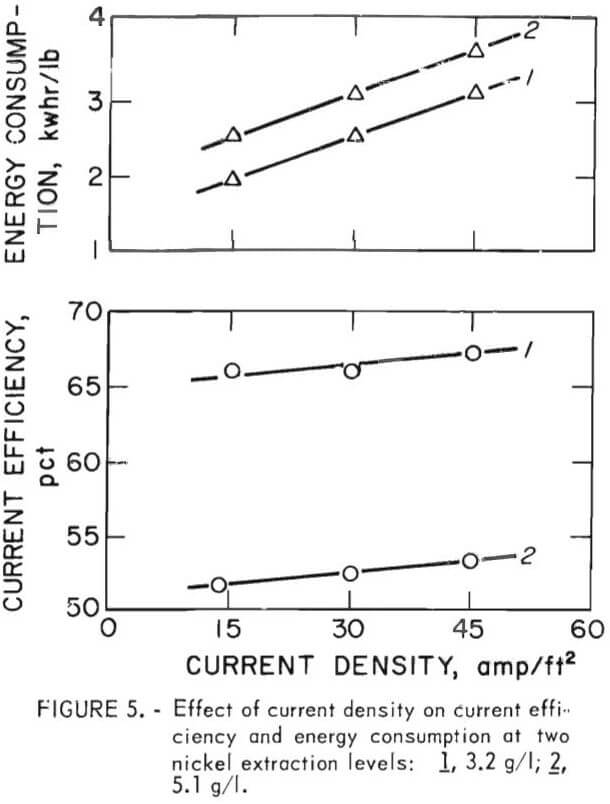 The determination of an optimum current density, which is essentially a production rate factor, is necessarily based on opposing cost factors beyond the scope of this preliminary investigation. These include capital outlays, operating costs, and the necessity of maintaining quality. Optimization, an interrelated cost minimum, can be approached by mathematical analysis using models. However, the rapid escalation of energy consumption tends to curb prospects of high current density operation in view of recent trends toward energy conservation.
The determination of an optimum current density, which is essentially a production rate factor, is necessarily based on opposing cost factors beyond the scope of this preliminary investigation. These include capital outlays, operating costs, and the necessity of maintaining quality. Optimization, an interrelated cost minimum, can be approached by mathematical analysis using models. However, the rapid escalation of energy consumption tends to curb prospects of high current density operation in view of recent trends toward energy conservation.
Effect of Temperature
The effect of increasing the electrolyte temperature was examined by conducting tests between 43° and 85° C, with other variables held constant at the levels described initially. Results showed over the temperature range used, the current efficiency pct (60 to 85 pct) and the energy consumption decreased 40 pct kwhr/lb Ni).
This decrease in energy consumption is due to generally known temperature effects and to buffering, which increase the bulk conductivity of the electrolyte and decrease the polarization of electrodes. For example, electrolyte containing 85 g/l Ni and 5.3 g/l H2SO4 exhibits, at 50° C, a pH of about 1.7 and a specific resistivity of 11.2 ohm-cm. When heated to 85° C the indicated pH is about 2.1 and the specific resistivity 8.0 ohm-cm. The temperature-associated changes in polarization and specific resistivity are reflected together in the voltage required to maintain a constant current flow. This is shown at cathode current densities of 20 and 30 amp/ft² in figure 7, both for an all NiSO4 electrolyte and also for an electrolyte containing 100 g/l Na2SO4. 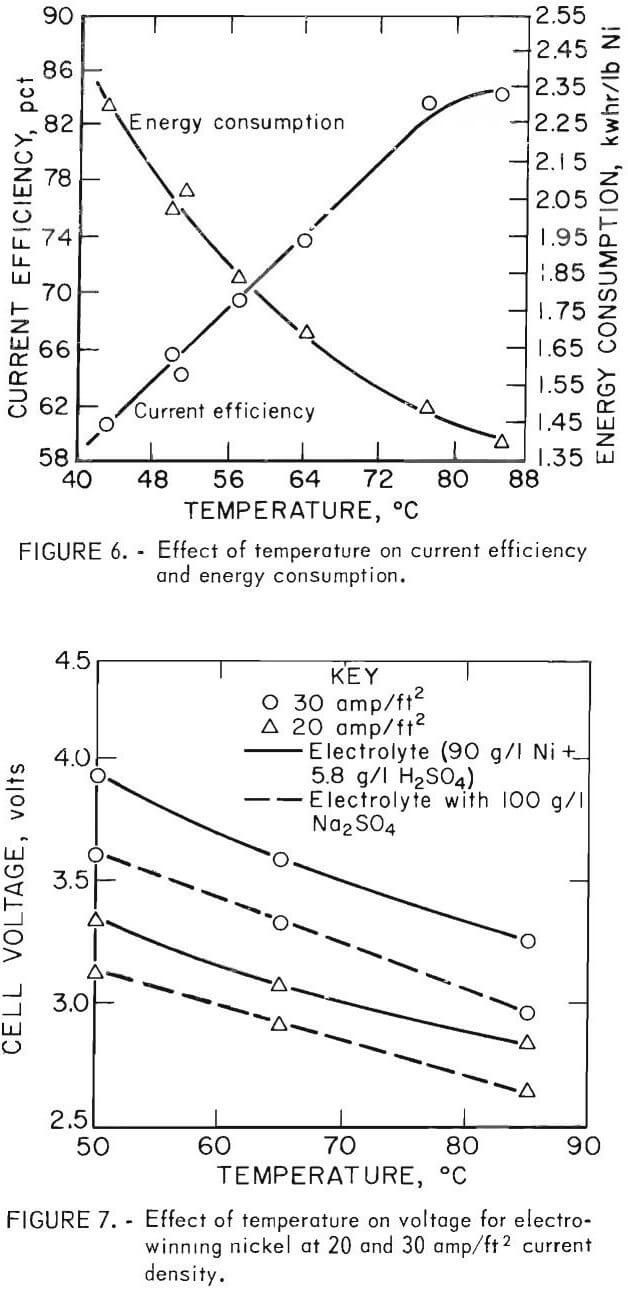 (The effect of Na2SO4 in the electrolyte is discussed in more detail in a later section.) It can be seen that the voltage is decreased about 0.25 volt either by increasing the temperature from 50° to 65° C or by adding Na2SO4 at the same temperature. Moreover, combining the temperature increase and the salt addition reduces the voltage by 0.5 volt, a greater energy saving. Thus temperature increases and salt addition offer electrowinning an independent means of reducing the energy consumption that would be particularly advantageous if waste plant heat from furnacing were available.
(The effect of Na2SO4 in the electrolyte is discussed in more detail in a later section.) It can be seen that the voltage is decreased about 0.25 volt either by increasing the temperature from 50° to 65° C or by adding Na2SO4 at the same temperature. Moreover, combining the temperature increase and the salt addition reduces the voltage by 0.5 volt, a greater energy saving. Thus temperature increases and salt addition offer electrowinning an independent means of reducing the energy consumption that would be particularly advantageous if waste plant heat from furnacing were available.
Comparison With Electrowinning in a Diaphragm Cell
Nickel was electrowon in a diaphragm cell to compare energy requirements and general procedures with those obtained with the nondiaphragm cell. Diaphragms are tightly woven fabric used to effect a separation of the cathode area, into which electrolyte is metered, from the anode area. Although electrolyte can seep through and the current can pass, hydrogen ions generated at the anode cannot diffuse back into the cathode region and be discharged, so comparatively large nickel extractions are obtained at very high current efficiency. But, because of so few hydrogen ions, sodium ions must be present as alternates to provide a supporting electrolyte of sufficient conductivity. In testing the diaphragm cell, conditions were nearly those of the nondiaphragm cell except for unavoidable differences owing to the cell characteristics. These differences include lower electrolyte flow rates to obtain extractions of 20 to 30 g/l Ni per pass. Also the addition of Na2SO4 and generally boric acid as well, constituted further variations.
Table 1 shows conditions and results obtained with the two cells as used for comparative purposes. The electrical energy consumption rate at 50° C was at first about 21 pct greater for the nondiaphragm cell; but, when Na2SO4 was then added to the electrolyte of the nondiaphragm cell, the difference was found to be only 4 pct. With the cells operated at 65° C, the energy consumption rate was just 5 pct greater in the nondiaphragm cell. After the salt addition it was about 5 pct less. Thus, the advantage of diaphragms decreased with increasing temperature to a modest value at 65° C. This is true if the extraction in the nondiaphragm cell is held to about 3.5 g/l Ni.
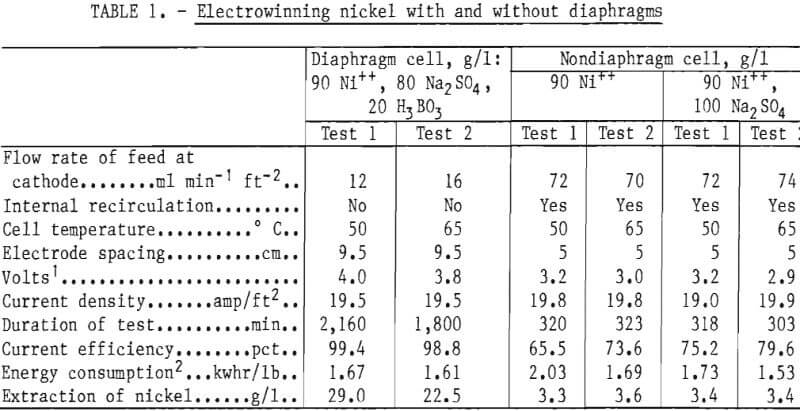
The advantage of large extractions is most important where electrolyte must be given extensive purification treatment between passes as, for example, when used alternately to leach a crude smelter product. By contrast, in solvent extraction-electrowinning, the electrolyte is only recycled through stripping and small extractions per pass are acceptable. They also lead to proper volume ratios of aqueous to organic phases (-1:1) in the mixers during stripping.
The advantage of the high current efficiency provided by diaphragms is partially offset by the higher cell resistance due to the diaphragms and to increased electrode spacing. The industrial energy consumption rate for nickel electrowon in diaphragm cells at 60° C is about 1.68 kwhr/lb Ni.
In general, results indicate that for solvent extraction electrowinning the nondiaphragm cell is preferrable since the added cost and size of diaphragm cells plus maintenance must be considered. Unresolved questions exist regarding the use of Na2SO4, but shakeout stripping tests with solvent used for nickel extraction have shown that it caused no significant impairment to stripping or to phase disengagement time. However, because of higher ionic strength, an undesirable increase in the slight solubility of organic phase in electrolyte may occur, which could have some adverse affect on electrowinning.
Effect of Additives to Electrolyte
Sodium Sulfate
The addition of Na2SO4 to electrolyte needs further consideration because of its long association with nickel plating and because no clear objection to its use with solvent extraction was established. In diaphragm cell operations it improves conductivity in the catholyte and helps maintain the quality of the deposit where there is only convective stirring. Concentration in the industrial operation at Outokumpu Oy, Finland, ranges between 120 and 210 g/l depending on the crystallizer operation. In plating baths it is thought to permit higher current density with better current distribution by depressing the ionization of Ni2SO4 solution. Thus the formation of knobs and sprues on the cathode are inhibited.
The beneficial effects of Na2SO4 additions include a lowering of bulk resistivity and an increase in pH, just as occurs with increasing temperature, as shown in table 2. A 100-g/l addition reduced specific resistivity about 25 pct at 65° C and increased the pH sufficiently to cause a calculated reduction in hydrogen ion activity of 21 and 35 pct for acid concentrations of 6.1 and 12.2 g/l, respectively. While this effect boosts current efficiency, it detracts from the aggressiveness of electrolyte to strip the organic material. Efficient stripping is essential to prevent nickel carryover into the copper circuit, so a compensating increase in the acidity of the system would result in practice.
The effect of 100-g/l Na2SO4 addition was tested at 50°, 65°, and 85° C for comparison with tests with no addition. Other conditions such as pH of the feed were the same. Results are shown in table 3. The decrease in energy consumption for nickel extraction of 3.3 g/l was 14.4 pct (0.29 kwhr/lb) at 50° C and 12.6 pct (0.22 kwhr/lb) at 65° C. Where extraction was only slightly increased to 3.7 g/l at 65° C, the decrease in energy consumption was only 6.4 pct (0.11 kwhr/lb) because of the competing conductive characteristics of the additional hydrogen ion. At 85° C and with an extraction of 4.2 g/l, the salt addition reduced energy consumption only 7.4 pct (0.1 kwhr/ lb). Other tests at this temperature, wherein extractions were increased to as high as 13 g/l Ni and current densities to 39 amp/ft², gave similar decreases of around 6 pct in energy consumption over comparable tests without salt.
Boric Acid
Boric acid has been used extensively in nickel plating and refining with additions of up to 30 g/l. Studies have indicated it increases cathode polarization and reduces stress in deposited metal by preventing absorption of colloidal particles that form in the region of high pH near the interface. Additions of 8 to 10 g/l were used for years in the diaphragm cells at Outokumpu Oy, Finland. No difficulties were encountered with as little as 4 g/l in the electrolyte.
Additions of boric acid to the synthetic electrolytes had no noticeable effect on pH but increased the bulk resistivity about 0.7 ohm-cm. Slightly lower current efficiency and higher energy consumption were obtained in tests conducted at 85° C when boric acid was added, as shown in table 4. In general, the use of boric acid in the nondiaphragm cell is considered unnecessary because of the low pH (<2.0) and the internal recirculation. The cathode metal obtained was consistently smooth and ductile, and it had a metallic sheen or luster whether boric acid was added or not.
Electrowinning at 85° C
Further electrowinning tests were conducted at 85° C in which factors other than temperature were varied to determine energy consumption rates under such conditions. In particular, current density and extraction per pass could be increased markedly without experiencing the rapid rise in energy consumption characteristic at lower temperatures. Data from a number of tests are compiled in table 4. The effects of first an increase in current density, then extraction, and then both are shown. It can be seen that electrical energy consumption under the latter conditions—test 5—was about the same as with the initial parameters, that is 2.02 kwhr/lb Ni. Tests 6 and 7, with boric acid added, show a slight increase in energy consumption over tests 4 and 5 (without boric acid but otherwise similar conditions), and test 8 shows the decrease resulting from sodium sulfate addition, which is about 6.8 pct.
Electrowinning at 85° C is shown to be a viable alternative, because (1) large extractions per pass could reasonably be adapted to solvent extraction by providing for aqueous recycle in the mixers to obtain a 1:1 aqueous to organic ratio during stripping and (2) high current density with large extractions would provide much of the heat through resistance heating of reduced volumes. The disadvantages include some limitations on construction materials; increasing corrosion to equipment, including anodes; and increasing evaporative heat losses with attendant misting and crystallization problems.
Electrowinning From Laterite Processing
The combining of electrowinning with the solvent extraction step developed for the Bureau process for low-grade laterites must be done in a manner compatible with the requirements relating to the overall process. Preferred flow and nickel transfer rates must be matched in the solvent extraction and electrowinning circuits. As a result, -1:1 aqueous to organic ratios are attained in the mixers during stripping.
To avoid excessive solution recycle, a 5-g/l or greater nickel extraction should be obtained from electrolyte as it passes through the cell. Increasing the extraction from 3.2 to 5.0 g/l in the experimental cell under the initial conditions increased the energy consumption about 15 pct. Increasing the temperature under the initial conditions to 65° C decreased energy consumption about 15 pct. Therefore, it was decided to increase the temperature to at least 65° C in initial operations during subsequent integrated circuit tests.
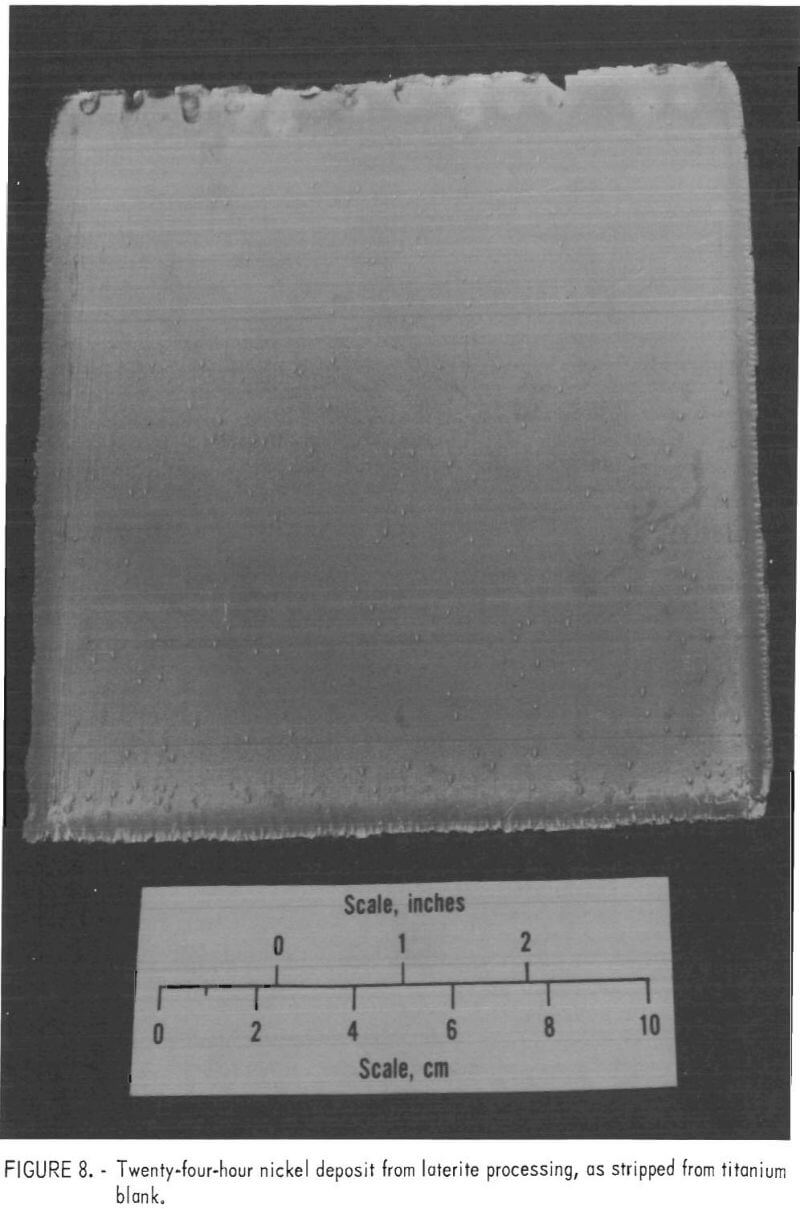
These parameters were tested in the experimental cell with electrolyte from the bench-scale solvent extraction unit while actual leach liquor was processed. The pregnant electrolyte from stripping contained 90 g/l Ni at pH 2.0. It contained about 1 g/l residual H2SO4. Continuous operation for 24 hours at 65° C with a cathode current density of 20.7 amp/ft² and an average extraction of 5.3 g/l Ni resulted in a current efficiency of 63.2 pct and an energy consumption of 2.10 kwhr/lb Ni. In a second test of 26 hours duration, with a cathode current density of 21.7 amp/ft² and an average extraction of 6.5 g/l Ni, the current efficiency was 57 pct and the energy consumption 2.3 kwhr/lb Ni. Thus, the energy consumption rate was held down and the parameters were considered satisfactory for pilot plant use. One of the products is shown in figure 8.
Experimental Cobalt Electrowinning
Cobalt electrowinning was patterned after the Boulder City, Nev., Experiment Station work where tests in nondiaphragm cells using cobalt sulfate electrolyte showed that current efficiency increased with concentration and temperature while energy costs decreased and plate character improved. Pilot plant work at Boulder City was conducted at 60° C because of rapidly increasing evaporation rate at higher temperatures and because higher zinc could be tolerated while producing good plate. Although tests at cobalt concentrations above 70 g/l Co appeared favorable, stronger electrolyte was not used because the preparation required filtration of nearly neutral solutions that became quite viscous at higher concentration. Since the filtration would not be required in a solvent extraction-electrowinning operation, higher concentrations could be preferable.
During the current investigation initial work with the experimental cell involved attempts to deposit cobalt on titanium starter blanks with synthetic electrolyte under a wide range of conditions. This was generally unsuccessful because the cobalt tended to loosen prematurely from the titanium, resulting in a thin, curled, or warped sheet. However, with type 316 stainless steel blanks that exceeded anode dimensions by a ¾-inch margin, uniform, adhering deposits were repeatedly obtained in tests of up to 49 hours. With plastic insulators placed on the edges of the stainless steel blanks, the cobalt could be easily stripped. On occasion, the stripped cobalt was returned to the experimental cell as a cathode. (See fig. 9.)
When cobalt is electrowon from electrolyte containing manganese, MnO2 is deposited on the anodes. When manganese is not present, a small amount of insoluble hydrated trivalent cobalt oxide is deposited on the anodes. This appears as a black, moderately compacted, amorphous material that tends to flake off. A washed and dried sample contained about 55 pct Co. As a result of apparent variations in the quantity of scale being formed, an effort was made to correlate test conditions with formation by collecting and noting the amounts. Results under various conditions of temperature, extraction, and current density are shown in table 5; however, because of the limited number
of tests, trends were not clearly established. There was some indication, based on discoloration of floating polypropylene balls in the cell, that at low temperature; the powder may have dispersed in the electrolyte rather than deposit on the anodes. The oxide is essentially insoluble in electrolyte and might therefore have to be removed periodically from the bottom of the cell by a vacuuming and filtering operation or by retention within anode bags.
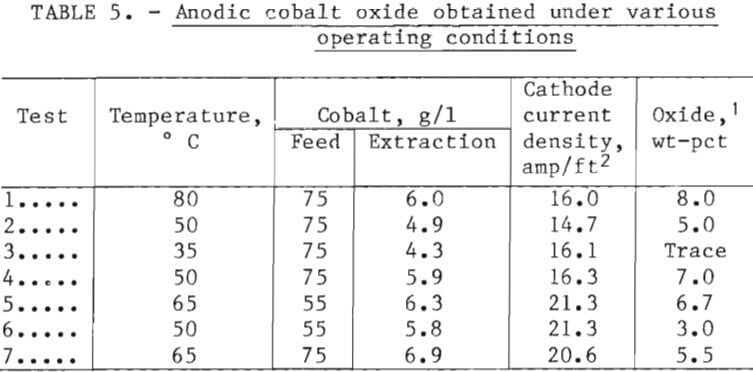
Cobalt differs from nickel in that the plate is rigid and brittle; therefore, if deposition on starter sheets is proposed, it is necessary that they be straight and easily stripped, a condition not easily obtained. The curling and warping of deposits observed in initial tests decreased markedly when stainless steel blanks were used; but, even so, survival following trimming and strapping would be low to nonexistent. In commercial operations in Katanga, Zaire, metal of 1 or 2 days deposition is roll-crushed, heat-treated under vacuum to remove hydrogen, tumble polished, and sold as broken pieces.
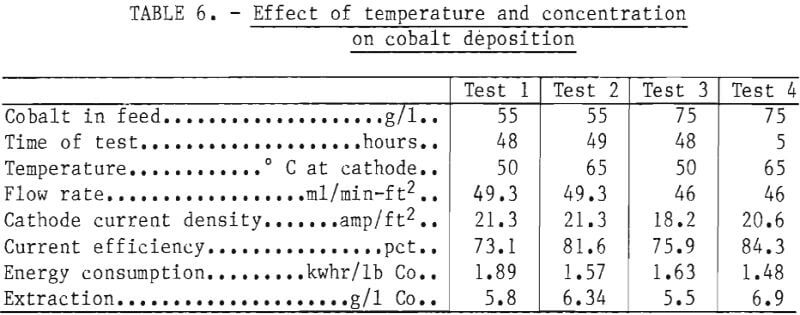
Numerous preliminary electrowinning tests were conducted, the most applicable of which are represented by the data from four specific tests of 5 to 49 hours duration summarized in table 6. These generally span practical limits for concentration and temperature for reasons similar to those for nickel deposition. In all four cases excellent sheets of satiny-white metal were produced that stripped readily from the stainless steel blanks. Even with a higher extraction, the power consumption was 22 pct lower with a concentration of 75 g/l Co and a cell temperature of 65° C than at 55 g/l Co and 50° C. Tests were also conducted at 85° C with electrolyte containing 87 g/l Co and a cathode current density of 35 amp/ft². With a cobalt extraction of 21 g/l, the current efficiency was still about 78 pct, and the electrical energy consumption around 1.5 kwhr/lb Co. Use of a high acid concentration (57 g/l) under these same conditions apparently helped control anodic cobalt oxide formation, since only 1.5 g was produced, but operation under these extreme conditions is considered impractical.
Conclusion
The investigation of the effects of variables affecting nickel electrowinning has shown that electrical energy consumption increases with increasing extraction and current density and decreases with increasing temperature and concentration of metal ions. The presence of Na2SO4, as required for diaphragm cell operation, also decreases energy consumption and would be recommended, except that compatibility with solvent ion exchange was not determined. With solvent extraction, the nondiaphragm cell is satisfactory. Specific conclusions as to optimum parameters were not reached because of opposing factors not subject to evaluation in this preliminary study and because of an interdependency with solvent extraction; however, electrowinning at a temperature of 65° C or above was found to provide suitable values for current efficiency and energy consumption with an extraction per pass of 5 g/l or better. Under these conditions nickel was electrowon at 2.10 and 2.30 kwhr/lb for extractions of 5.0 and 6.5 g/l, respectively, in tests of about 24 hours’ duration with electrolyte from laterite processing.
Under conditions similar to those for the nickel electrowinning, cobalt was electrowon with lower electrical energy consumption rates and higher current efficiencies than nickel, so that recommended pilot plant parameters for a 5-g/l extraction per pass were 50° to 65° C with a cobalt concentration of 55 to 75 g/l in the cell.
As part of an overall goal to develop technology that can maintain an adequate supply of minerals to meet national economic and strategic needs, the Bureau of Mines is evaluating a method for selectively extracting nickel and cobalt from low-grade domestic laterites. The method involves electrowinning to recover the metals in pure form; therefore, preliminary laboratory-scale electrowinning tests were conducted in both a nondiaphragm and a diaphragm cell to determine the effect of variables on energy efficiency under controlled conditions. Electrolytes were generally reagent-grade sulfate solutions that were prepared to simulate electrolyte that could be obtained by stripping nickel- and cobalt-rich solvent extraction reagents. Limited tests were conducted with electrolyte generated from laterite leach solutions.
Electrical energy consumption in a nondiaphragm cell was 2.06 kwhr/lb Ni with the cell operated at 50° C, a cell feed of 90 g/l Ni, a pH of 3.5, an extraction of 3.2 g/l, and a cathode current density of 20 amp/ft². The energy requirement decreased with increasing concentration and temperature, and increased with increasing, extraction and current density. At 50° C, energy consumption in the nondiaphragm cell was 21 pct greater than in the diaphragm cell, but it was only 5 pct greater at 65° C. For this reason and because of overall cost considerations, operation at 65° C with the nondiaphragm cell was preferred.
Smooth sheets of cobalt were obtained by electrowinning in a nondiaphragm cell at selected temperatures from 50° to 85° C and with cobalt concentrations from 55 to 87 g/l. The energy consumption (1.48 kwhr/lb) was 22 pct lower with a cobalt concentration of 75 g/l and a cell temperature of 65° C than with a cobalt concentration of 55 g/l and a cell temperature of 50° C.
