Table of Contents
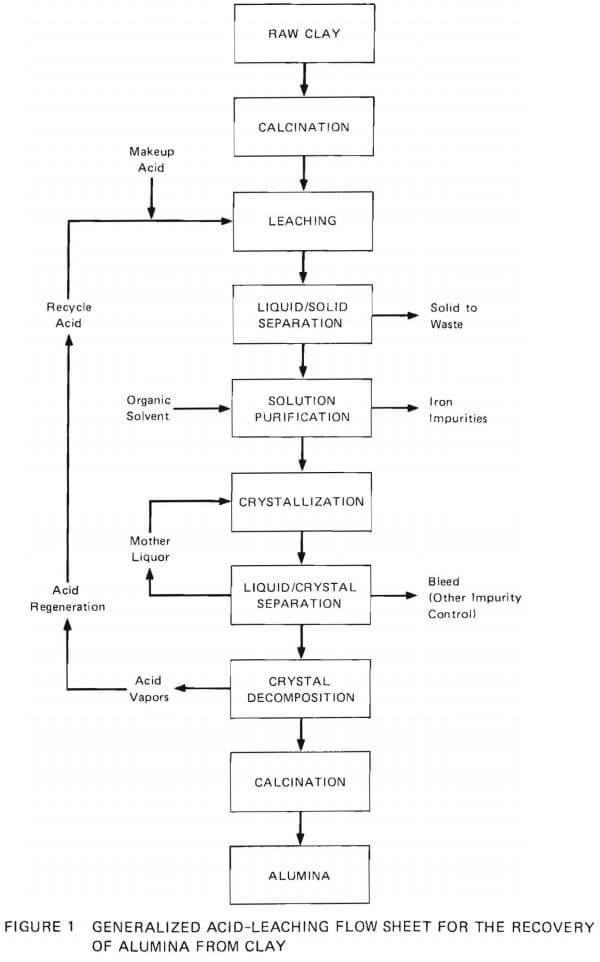
The currently proposed method for the recovery of alumina from nonbauxitic materials involves acid leaching (Figure 1). Primary emphasis is on kaolin clays. To date, leaching by nitric acid, hydrochloric acid, and sulfurous acid has been considered, and it appears that leaching with hydrochloric acid (25-27%) is the most practical process. The first step in the overall process (Figure 1) is to decompose the hydrated aluminum silicate ore to meta kaolin (silica and acid-soluble alumina) by calcination at 700° to 800°C. This calcined materials is then leached with HCl to extract aluminum as dissolved Al3+. The undissolved solids are separated from the process stream, and the solution is purified of iron by organic solvent extraction. The aluminum content of the fluid is then precipitated as AlCl3·6H2O by evaporation or by sparging the solution with HCl gas. Then the aluminum chloride hexahydrate is decomposed into alumina and HCl by calcining at high temperature. This process releases HCl, which is then recirculated for leaching of fresh ore and for crystallization.
The thermal decomposition of aluminum chloride hexahydrate is energy intensive. Hence, an alternative process in which aluminum hydroxide or oxides are precipitated from the aluminum chloride solution, if realizable, would be a technically and economically better alternative.
Hydrothermal hydrolysis is a chemical process whereby the oxide or hydroxide of a metal is produced from an aqueous solution by an increase
in temperature. Water is consumed and acid (H+ ion) is produced. In chemical terms, this phenomenon may be represented as
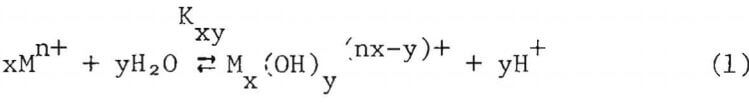
with y = nx for the overall precipitation of the neutral solid product. Because the equilibrium constant Kxy increases with temperature, precipitation of Mx(OH)y will occur when a solution of the ion Mn+ is sufficiently heated. However, the process is much more complex than indicated by the simple equilibrium statement in Equation 1, and the yield of oxide or hydroxide obtained in a practical hydrothermal hydrolysis recovery technique will depend on the kinetics of nucleation and growth of the solid phase, as well as on the thermodynamic properties of the system.
Recovery of alumina by hydrothermal hydrolysis would be part of a process that makes use of the currently proposed calcination and hydrochloric acid leaching of the ore. However, instead of using HCl gas to induce AlCl3·6H2O crystallization from the pregnant liquor, this liquor would be heated in an autoclave during which boehmite (y – AlOOH) would be precipitated by hydrothermal hydrolysis. Because fewer steps would be required in the overall process, and because hydrothermal hydrolysis can be carried out at relatively low temperatures with modest energy consumption, hydrothermal hydrolysis may represent a better alternative process step to recover alumina from aluminum chloride solutions.
D. Macdonald et al. have demonstrated the feasibility of boehmite precipitation by hydrothermal hydrolysis of dilute aluminum chloride solutions. However, this work was restricted to a study of the thermodynamic properties of the precipitation process, and no work was done on the kinetics of the reaction. Furthermore, the Al3+ concentrations used were well below those envisaged for a practical boehmite recovery process. Hence, the U.S. Bureau of Mines contracted with SRI International to study the feasibility of boehmite precipitation from stronger aluminum chloride solutions representative of process streams. The study is in three sequential phases: (1) thermodynamics of boehmite precipitation, (2) kinetics of precipitation, and (3) investigation of the feasibility of a continuous flow-through system.
This report describes the results of Phase I. During this period the extent of boehmite precipitation from dilute aluminum chloride solution (5 wt%) representing the concentration of AlCl3 in the end liquor from AlCl3·6H2O crystallization and from concentrated AlCl3 solutions (30 wt%), representing leach liquor, was investigated as a function of hydrolysis temperature. The effect of added HCl acid on the boehmite precipitation was also studied. We have, with limited success, attempted to measure the extent of hydrothermal hydrolysis by means of a high temperature pH cell.
Experimental Methods for
Hydrothermal Precipitation of Boehmite
from Aluminum Chloride Solutions
High Temperature pH Cell
The principal objective of Phase I has been to study the thermodynamics of boehmite precipitation from both weak and concentrated aluminum chloride solutions (including pure solutions and solutions containing impurities) as a function of hydrolysis temperature, aluminum ion concentration, and pH of starting solution and impurities. The overall chemical reaction involved in boehmite precipitation can be written as
Al3+ + 2H2O → AlOOH + 3H+………………………………………….(2)
Thus, hydrogen ions are generated during hydrothermal hydrolysis, thereby decreasing the pH of the solution. By monitoring that pH, the extent of hydrolysis can be estimated knowing the hydrolysis species involved. For this purpose, a high-temperature pH cell similar to the one employed by D. Macdonald et al. was constructed.
The cell consists of a 250 ml, PTFE-lined stainless steel autoclave purchased from Bergholf America Inc., Raymond, N.H. The PTFE, made by a special isostatic compression process, offers properties of chemical inertness, mechanical strength, temperature resistance to 250°C and uniform thermal expansion which make it a good liner material for heating corrosive solutions. The experimental solutions were contained in a PTFE liner that fits within the stainless steel body. The top of the lid is also lined with a tight fitting PTFE insert. A PTFE 0-ring seals the lid and the body of the autoclave. The design minimizes the exposure of metallic parts of the autoclave to the corrosive solution. Details of the high temperature pH cell are shown in Figure 2.
The pH of the experimental solution was calculated from the potential difference between two reversible hydrogen electrodes, one immersed in the experimental solution and the other, in a reference solution. The
reference solution was contained in an inner compartment machined from a PTFE bar to a wall thickness of less than 1.5 mm. This thickness allowed rapid thermal equilibrium between the two compartments when the solution was heated. PTFE-covered magnetic stirrer bars were used in both compartments to agitate the solutions. The reference compartment was fastened to the autoclave lid by means of a tantalum collar and tantalum screws. A 0.76 mm diameter hole in the upper wall of the reference compartment above the liquid level allowed rapid pressure equilibrium, yet was small enough to minimize distillation from one compartment to the other prior to thermal equilibrium.
The reversible hydrogen electrodes consisted of pure platinum tubes (3 mm O.d., 1.65 mm i.d.) which were insulated from the autoclave and from each other by Conax fittings containing PTFE inserts. Before use, the electrodes were lightly platinized by electrolysis in a dilute solution of chloroplatinic acid. Thermocouples inserted inside the platinum tubes were used to monitor the temperature of the two compartments.
The liquid junction between the two compartments consisted of a porous PTFE disk held in a Rulon insert, which was then pressed into the bottom of the reference compartment. The PTFE disk was treated with a commercial sodium solution to make its surface wettable, then the disk was repeatedly boiled and cooled in saturated KCl solution. During boiling, air was expelled from the pores in the disk and replaced by the KCl solution when cooled. The liquid junction resistance was found to be approximately 1000 ohms, which did not provide a high liquid junction potential.
The autoclave was heated by a ceramic band heater. A chromel-alumel thermocouple sandwiched between the heater and the autoclave controlled the heater, while temperatures of the solutions were only monitored by the thermocouples inserted into the platinum tubes. The potential difference between the electrodes was measured by a digital voltmeter having an input impedance of 10 Mn.
Solutions of Al3+ in HCl-KCl media, where the concentration of chloride ion was held constant at 1 M, were prepared by dissolving AlCl3·6H2O and KCl in water, then adding HCl. In a typical experiment,
140 ml of this aluminum solution was placed in the outer compartment, while the reference compartment contained 15 ml of HCl-KCl solution with pH = 3 and [Cl-] = 1 M. To eliminate the presence of air before the solution was heated the autoclave was degassed by cyclic compression/ decompression with pure hydrogen about five times. Then the autoclave was filled with 200-psi hydrogen. After a steady state potential difference was obtained, the solutions were heated to a higher temperature.
Boehmite Precipitation Experiments
The extent of boehmite precipitation was investigated as a function of temperature, time and added acid. For this purpose, 100 ml of aluminum chloride solution was heated in the PTFE-lined autoclave for a predetermined time and then the autoclave was quenched. The precipitate was filtered in a fine-fritted glass funnel, dried at 120°C, and weighed. A clear filtrate was obtained.
The filtrate was analyzed for aluminum by back-titrating in EDTA with ZnS04, using Super Eriochrome Black T indicator. Aluminum concentrations in the solutions were also analyzed by atomic absorption spectrometry. The titration and atomic absorption results generally agreed within 10%.
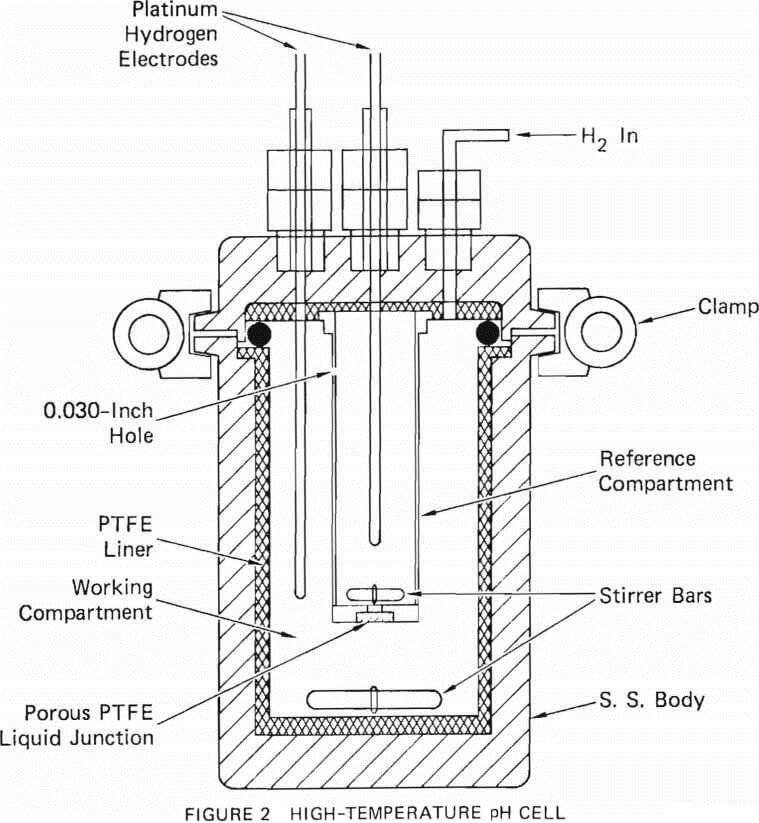
The PTFE-lined autoclave could not be used for boehmite precipitation experiments at temperatures higher than 250°C, nor can other commercially available polymers. A metal vessel was necessary to contain the solution at higher temperatures. The metals that can withstand hydrochloric acid at very elevated temperatures are tantalum, tungsten, molybdenum, and zirconium. Tantalum crucibles and liners are quite expensive. However, a molybdenum crucible of 4-cm diameter x 15-cm height was available at SRI. A molybdenum flange of 6.35-cm diameter x 0.32-cm thickness was electron-beam welded to the top of the crucible, and the crucible was covered with a 0.32-cm thick molybdenum plate. A gold wire gasket (0.64-mm initial diameter) acted as a seal between the lid and the crucible. The crucible illustrated in Figure 3 is sealed with a stainless steel clamp having six lock screws that clamp the lid to the flange. For the experiments, typically 100 ml of aluminum-bearing solution was sealed inside the crucible. The sealed crucible was immersed in water inside a 2l Hastelloy C autoclave. The autoclave was heated to the desired temperature for the selected time and cooled. The solution inside the molbydenum crucible was filtered and analyzed for aluminum.
Results and Discussion
High Temperature pH Measurements
The high temperature pH cell can be represented as
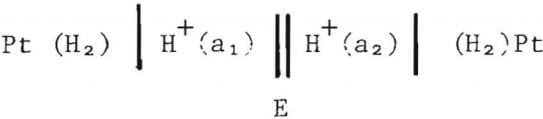
where subscripts 1 and 2 refer to the inner and outer compartments, respectively, and E is the potential difference between the inner and outer compartment platinum electrodes. Since the pressure of hydrogen is the same in the two compartments, the potential difference, E, is given by

where Ej is the liquid junction potential. D. Macdonald et al. have shown that in a weak aluminum chloride solution containing Al3+, Cl-, H+, OH , and K+ the liquid junction potential would be less than 1 mV, which represents a minor correction to the measured potential difference.
Denoting pH = -log (H+), Equation (4) can be rewritten as
pH2 = pH1 – F(E – Ej)/2.303 RT + log Y2/Y1……………………………………..(5)
where y2 and y1 are activity coefficients for hydrogen ion in the outer and inner compartments respectively. D. Macdonald et al. have shown that in dilute solutions following the Debye-Huckel model the activity coefficient term would make pH2 in Equation (5) smaller by an amount less than 0.01, and the activity coefficients can be ignored. Hence the pH of the outer compartment can be written as
pH2 = pH1 – F(E – Ej)/2.303 RT………………………….(6)
The above simplified relation is applicable only to dilute solutions. For solutions containing significant amounts of AlCl3, activity coefficients should be calculated and Equation (5) should be used.
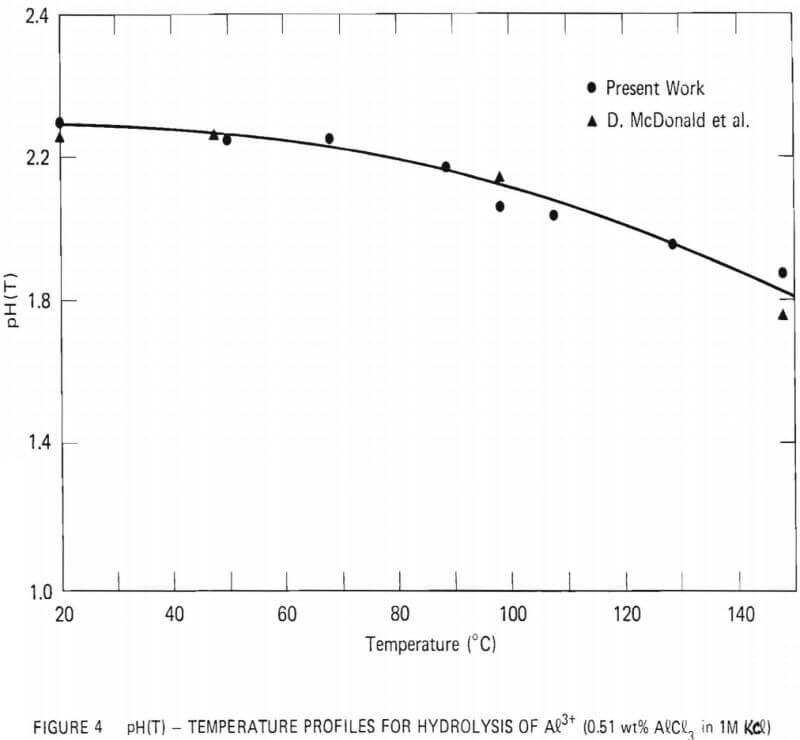
The high-temperature pH cell constructed for this study was similar to the one employed by D. Macdonald et al. During initial runs, a solution of 0.04-M Al3+ with 1.0-M Cl- was placed in the outer compartment while the reference solution was 1.0-M Cl- at pH = 3. The pH of the outer compartment as a function of temperature was estimated by measuring the potential difference between the platinum electrodes at various temperatures. The results are shown in Figure 4, along with the values reported by D. Macdonald et al. Excellent agreement between the two sets of values was observed.
However, during runs with a solution of higher aluminum concentration (corresponding to 5% AlCl3), numerous difficulties were encountered. Specifically, the potential difference between the platinum electrodes decreased with time at temperatures above 140°C. If the pH of the reference solution remains constant at a constant temperature, the hydrolysis of aluminum in the outer compartment will lead to increasing potential difference between the electrodes. Hence a decreasing potential difference with time at constant temperature implies that either the pH of the reference solution is decreasing or the aluminum ion hydrolysis is reversing. On cooling, it was found that the pH of the reference solution had indeed changed during the experiment from 3 to approximately 2.5.
The change in the pH of the reference solution can be caused either by diffusion or mixing of the inner and outer compartment solutions through the liquid junction or by vapor transport of the generally more acidic outer compartment solution. Since changes in the pH of the reference compartment continued to be observed in experiments where the pressure equilibration hole (0.75-mm diameter) was covered with a thin tape of Teflon, it was concluded that diffusion or a leak through the liquid junction was responsible for the change in pH. This was later confirmed by the presence of chloride ion in the reference compartment when a solution of nitrate was placed in the reference compartment, while a chloride solution was kept in the outer compartment. Even though
the porous PTFE disk is tightly secured to the reference compartment at room temperature (leak rate = 0.05 ml/hr/cm head), the disk may contract differentially compared to the reference compartment at elevated temperatures, allowing the solutions to mix.
Several alternative designs were tried in a major effort to improve the pH cell. This unsuccessful effort was a substantial fraction of the Phase I work. The porous PTFE disk was replaced by a porous zirconia disk and then by a porous zirconia rod. The zirconia was wettable, so that a reliable electrochemical connection could be made. However, the leak rate through the porous zirconia, even at room temperature, was sufficiently high to allow the liquid in the reference compartment to be pumped out by the centrifugal action of the stirrer bar in the outer compartment. Although addition of a baffle between the liquid junction and the stirrer minimized this pumping, at elevated temperature some mixing between solutions of the two compartments continued to occur.
Operation of the cell above 200°C increased the difficulties. During one operation the PTFE disk (liquid junction) separated from the body of the reference chamber. Both the disk and the reference chamber showed substantial changes in their dimensions. These difficulties, plus the observation that no boehmite precipitation occurred from 5 wt% AlCl3 solution at temperatures below 200°C, forced us to abandon the operation of a high-temperature pH cell and concentrate on a study of boehmite precipitation as a function of time, temperature, and HCl initially added.
Boehmite Precipitation Experiments
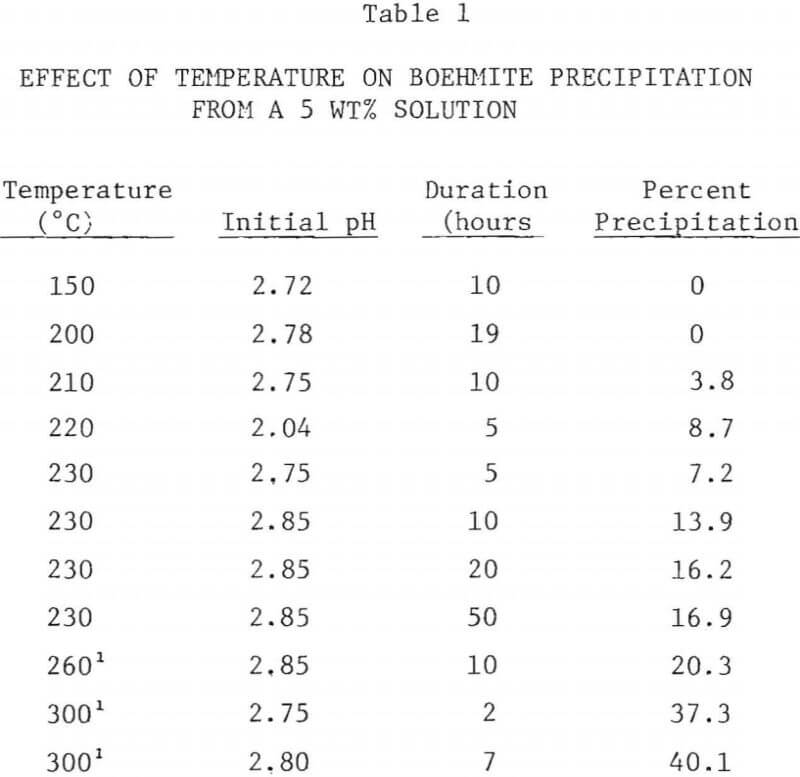
Boehmite precipitation experiments were carried out with 5 wt% AlCl3 and 30 wt% AlCl3 solutions in the temperature range of 150°C to 300°C. The PFTE-lined autoclave was used below 250°C and above that temperature the molybdenum crucible was used. X-ray diffraction analysis showed that the precipitate formed in this temperature range from aluminum chloride solutions was boehmite, yAlOOH (ASTM 21-1307). In runs with the molybdenum crucible, small amounts of a brownish deposit, presumably molybdenum oxide or hydroxide, were observed. A similar deposit was also found on the outside of the molybdenum crucible. It was likely that at the elevated temperatures molybdenum was slightly oxidized to oxides or hydroxides by water.
The precipitated boehmite was fine and granular and settled easily. However, a certain amount of precipitate tended to stick to the PTFE liner and to the stirrer bar, requiring scraping. The extent of boehmite precipitation was calculated from the EDTA analysis of aluminum content in the original solution and in the filtrate.
% precipitation = [Al]initial – [Al]Filtrate/[Al]initial………………………(7)
The results of boehmite precipitation experiments are shown in Tables 1-3.
Effect of Temperature
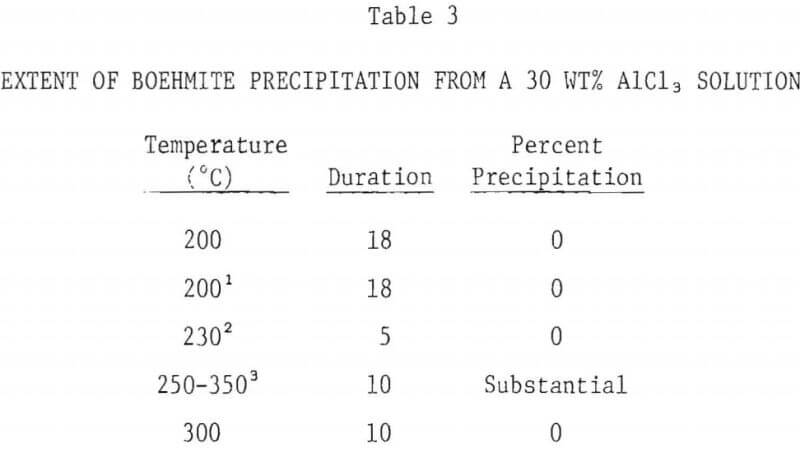
The extent of boehmite precipitation from a 5 wt% AlCl3 solution as a function of temperature after a period of 10 hours is shown in Figure 5. Below 200°C, no precipitation was observed, even after 20 hours. Above 200°C, the amount of precipitation sharply increased with hydrolysis temperature. At 300°C about 40% of the aluminum in the starting solution was precipitated.
The precipitation of boehmite from concentrated AlCl3 solutions (30 wt%) was not generally observed even at 300°C. However in one experiment, when an alumina thermowell broke during the run at night, releasing the gases and increasing the temperature of the solution to 250-300°C, substantial amounts of boehmite residue was found. The precipitate was obtained by redissolving the evaporated residue in water and by filtering. Vapor venting experiments above 250°C were not conducted due to temperature limitation of PTFE lined autoclave.
Effect of Time
The extent of aluminum removal from a 5 wt% AlCl3 solution was investigated as a function of hydrolysis time at 230°C. The results are shown
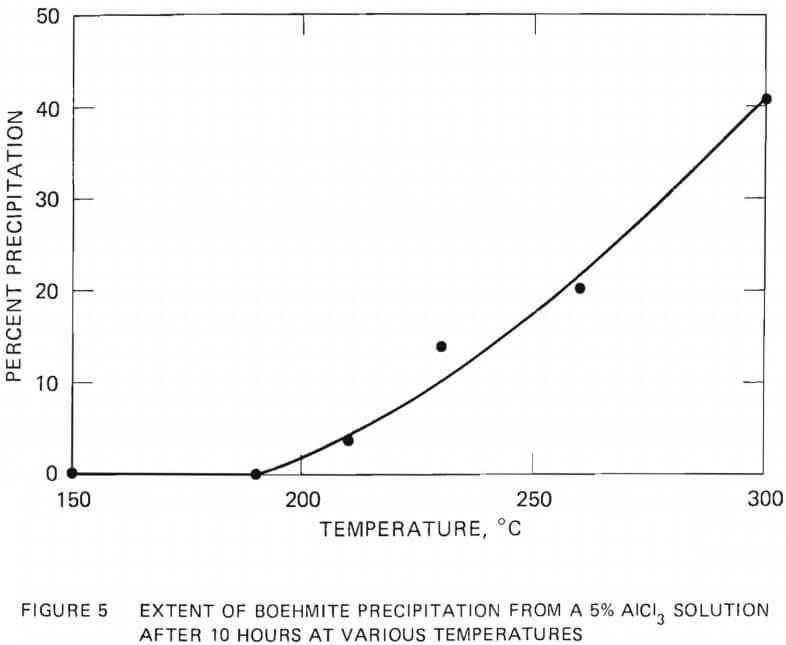
in Figure 6. The solution was rapidly heated to 230°C (about 40 minutes), held at that temperature for the selected time, and quenched rapidly in a flowing stream of water. Quenching was not possible with the experiments conducted in the molybdenum crucible above 250°C.
The rate of boehmite precipitation was found to be slow at 230°C, requiring several hours to obtain noticeable precipitation. The amount of precipitate increased with hydrolysis time up to a period of 10 hours, after which the precipitation rate was very slow. No substantial increase in the amount of precipitation was observed when the hydrolysis time was extended from 20 hours to 50 hours.
At higher temperatures the precipitation was more rapid. At 300°C the amount of boehmite formed after 7 hours was substantially the same as the amount at 2 hours (see Table 1). The rather slow heating and cooling of the 2-liter autoclave used for these experiments prevented obtaining meaningful results for periods shorter than two hours.
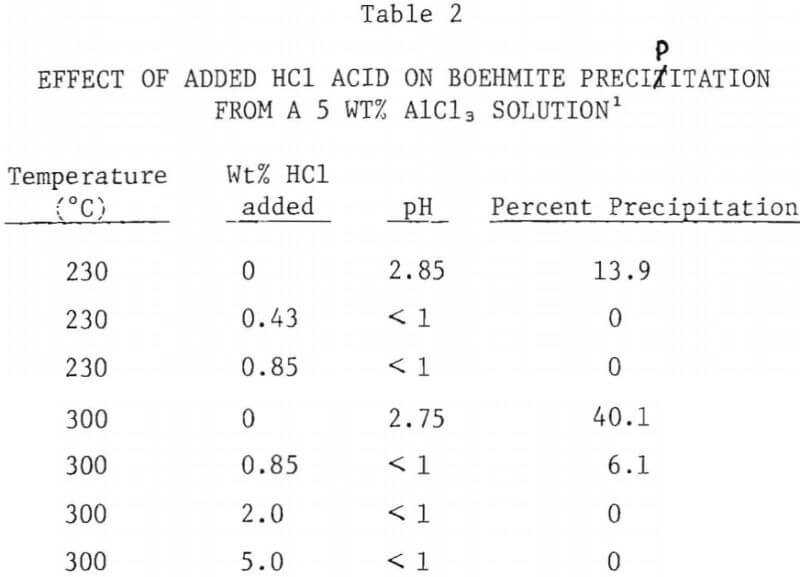
Effect of Added HCl Acid
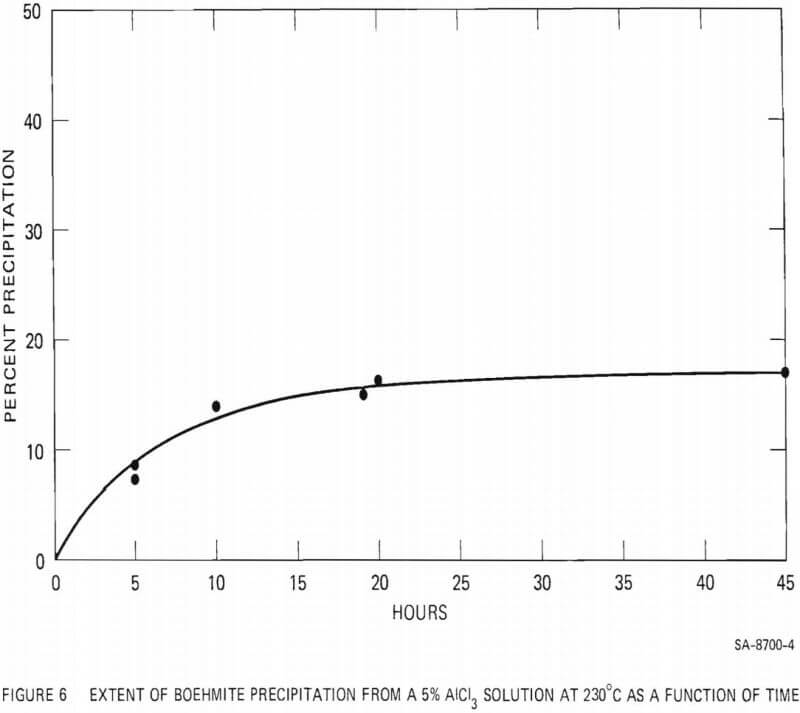
The effect of added HCl acid on the extent of boehmite precipitation from a 5% AlCl3 solution was investigated at 230°C and at 300°C. The results are summarized in Figure 7. Addition of HCl acid to the AlCl3 solution drastically reduced the amount of precipitation. At 230°C addition of less than 0.5 wt% acid prevented the precipitation of boehmite during a period of 10 hours. At 300°C, addition of 0.85 wt% acid decreased boehmite yield from 41% (with no excess acid) to 6%. With acid addition above 2% no precipitation was observed, even at 300°C.
Since earlier experiment had shown no precipitation with 30% AlCl3 solution even at 300°C the effect of additional acid on that solution was not investigated.
The reactions involved during aluminum ion hydrolysis in aqueous solutions at elevated temperature can be written as
2Al 3+ + 2H2O = Al2(OH)2 4+ + 2H+………………………………………………(8)
14Al3+ + 34H2O = Al14 (OH)8+34 + 34 H+……………………………………..(9)
followed by the precipitation of boehmite according to
Al14 (OH)8+ 34 → 14Al OOH + 6H2O + 8H+……………………………(10)
These sets of reactions were found to be in agreement with high-temperature pH measurements in dilute Al3+ solutions. D. Macdonald et al. have also shown that at temperatures below 125 °C the principal species involved in aluminum ion hydrolysis are Al3+ and the dimer Al2(OH)2+. At higher temperature the large polymer Al14(OH)8+ becomes important. They postulated that the large polymer is the precursor to the formation of boehmite.
It should be emphasized that other hydrolysis species, such as Al3(OH)4+, have been proposed. Further, there may be chloride or oxychloride complexes at high aluminum chloride concentration about which there is no reliable information.
The hydrolysis of aluminum ion generates 3 H+ ions for each Al3+ ion. As the H+ ion concentration increases because of hydrolysis, the rate of hydrolysis will slow if the H+ ions generated are not removed.
In highly acidic solutions the Al3+ species may be more stable than the hydroxide complexes, so that no precipitation will occur. This hypothesis is consistent with the observation that the addition of HCl acid to the aluminum chloride solution drastically reduces the amount of precipitate formed.
An alternative explanation for the small amount of precipitate formed involves the kinetics of precipitation. It is likely that even though hydroxide complexes exist as a result of hydrolysis, the nucleation and growth of boehmite precipitate may be inhibited in highly acidic solutions. Since the high-temperature pH cell was not functional over 200°C, it was difficult to ascertain the extent of hydrothermal hydrolysis from weak and concentrated aluminum chloride solutions.
Effect of Impurities
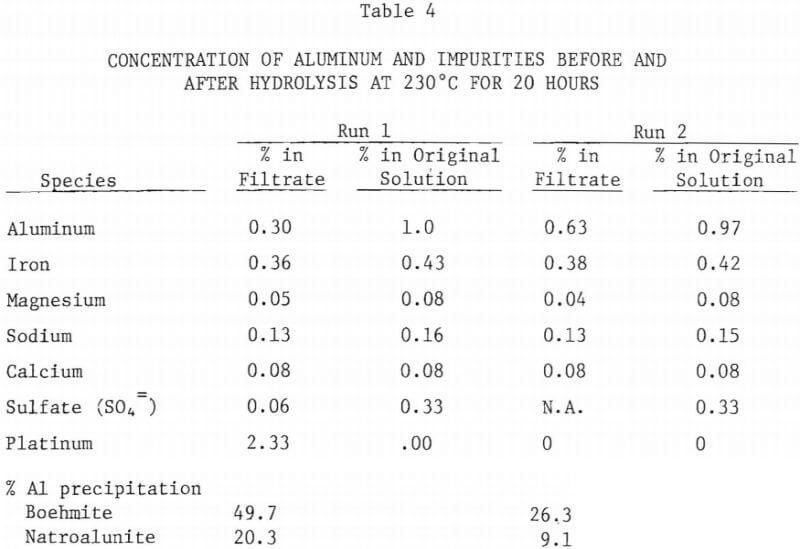
Since the leach liquor will contain impurities such as iron, potassium, calcium and magnesium, the effect of these impurities on the amount of boehmite precipitation was investigated. For this purpose small amounts of FeCl2 , Na2SO4, CaNO3·4H2O, and Mg(Clo4)2 were added to nominal 5% AlCl3 solution. The solution was heated to 230°C for 20 hours. During this run, presumably due to presence of nitrate ion along with chloride ion, platinum thermowell partially dissolved during hydrothermal hydrolysis. However, the amount of aluminum precipitated (70% Al) was much higher than in similar runs with no impurities added. Table 4 lists the concentration of aluminum and other elements before and after hydrolysis. The precipitate was identified to be mostly boehmite with minor amounts of natroalunite. In a second run the platinum thermowell was protected with a PTFE liner and the experiment was repeated under similar conditions. The amount of precipitation (35.4%) was smaller than the earlier run, but it is still higher than when no impurities were added.
The enhanced precipitation of boehmite occurring when certain impurities were added supports the earlier mentioned hypothesis that stable hydroxide complexes are formed during hydrolysis. Such impurities as sodium alunite, formed during hydrolysis, may act as a seed for further precipitation of boehmite. Investigation is required to identify the critical amount and nature of the impurity(s) that trigger boehmite precipitation.
Conclusions
- The precipitation of boehmite from 5% AlCl3 solutions is negligible below 200°C. Precipitation increases with increasing temperature, approaching 40% at 300°C. No precipitation occurs with 30% AlCl3 solution, even at 300°C.
- Addition of HCl acid to the 5% AlCl3 solution, even in small quantities, drastically reduces the amount of boehmite precipitation.
- Solution impurities such as Fe, Ca, Na, Mg, and Pt substantially promote the precipitation of boehmite. Additional investigations are needed in this area to identify the type and concentration of the impurities that promote the precipitation.
- Removal of HCl gases during hydrothermal hydrolysis at 250-300°C may facilitate precipitation of boehmite from concentrated solutions.
