Table of Contents
Among the significant technological accomplishments of the third quarter of the 20th century was the wide application of vacuum-to-metal melting. Many of the more common metals, as well as superior alloys of steel, have benefited from vacuum degassing, and the production of reactive and refractory metals leans heavily on vacuum-melting techniques. Fabrication and other forming operations of vacuum-melted material, carried out in air, were believed to be detrimental to the physical and mechanical properties of the finished material because of interstitial elements acquired during the forming operations.
In-Fab, a large inert atmosphere room containing forging and rolling equipment, was built in the early 1960’s through Government-industry cooperation to alleviate the problems of air fabrication. The technique was never found to be acceptable commercially in the United States. Limited studies of fabrication in a vacuum were conducted in the fabrication of refractory and nuclear metals. A common problem in these studies was the unexpected bonding of metal when ultraclean surfaces were present in the vacuum.
The Bureau of Mines, U.S. Department of the Interior, conducted an investigation to determine whether the solution to this problem could be applied to obtain bonds between scarce and critical metals and a noncritical substrate. This method could then be used to produce thin-clad hardware, conserving chromium, nickel, and cobalt, and help achieve the Bureau’s goals to minimize the requirements for scarce or critical minerals through conservation and substitution.
This report presents a review of the literature on vacuum rolling, a description of the equipment developed, experimental data obtained to date, and a brief discussion of the potential of vacuum rolling for eventual commercial adoption.
Metal Hot Rolling Equipment
Worldwide reviews of the vacuum-rolling equipment are given by Krupin and Yudina and by Krupin. Beginning with the first vacuum hot rolling mill in 1953, a number of mills of increasing size and capability were developed in the U.S.S.R. The U.S.S.R. mills can be evacuated to about 1 x 10 -6 torr and can roll at a maximum temperature of 900° to 1,600° C. The first two vacuum-rolling units, built by the Technical Institute of the Academy of Sciences of the U.S.S.R., were described by Amonenko in 1960. The first consisted of a small rolling mill with work rolls 85 mm in diameter and 150 mm long. The entire mill and frame, except for the drive motor and drive gears , were located within the vacuum chamber. From experience gained with this unit, a second vacuum rolling mill was designed and constructed. This second design incorporated the “roll-chamber” concept that became standard with U.S.S.R. vacuum-rolling technology. In this design, by constructing the chamber as an integral part of the mill housing, only the rolls were enclosed in the vacuum chamber. The volume and pumping capacity required were thus minimized. The screwdowns, roll bearings, gears, etc., were outside the chamber. The first mill of this type utilized rolls 200 mm in diameter and 300 mm long with a removable barrel to facilitate roll changing. Additional vacuum chambers, attached to each side of the mill chamber, enclosed manipulators and molybdenum- wound resistance furnaces capable of 1,500° to 1,600° C. This mill was later improved, as described by Smirnov, and installed at the Ukrainian Scientific Institute of Metals. Krupin and Pavlov described another “roll- chamber” vacuum rolling mill at the Moscow Institute of Steels and Alloys capable of operating at 5 x 10 -5 torr. The rolls were 210 mm in diameter and 340 mm long. Krupin also mentioned a two-high mill with 210-mm-diameter rolls that was enclosed in a vacuum chamber at the Leningrad Molding Institute of Kolinin before 1964. This mill was later converted to a “roll-chamber” type capable of 1 x 10 -4 torr. Problems with manipulators including overheating when operating through the furnace were mentioned.
The Technical Institute of the Academy of Sciences of the U.S.S.R. built another two-high vacuum rolling mill of the “roll-chamber” type, reported by Sigalov and Exerskii in 1968. This two-high mill utilized 400-mm-diameter by 450-mm-long rolls , a 150-kw drive motor, and was capable of 10 -5 torr. In 1961, the Physicotechnical Institute built a vacuum rolling pilot plant with six-roll stands to produce sheet and rods from chemically active metals at 1 x 10 -6 torr , mentioned by Ivanov. At the All-Union Research Institute, Tsygankov and Perfil’ev reported in 1961 on a fully automatic vacuum rolling plant for tube production. In 1977, Kolikov found that during helical rolling of refractory metals the billet could be reduced farther before fracture of the axial zone when rolling in vacuum than when rolling in air.
Another vacuum-rolling train, utilizing 210-mm-diameter rolls and a vacuum of 5 x 10 -5 torr, was set up at the Moscow Institute of Steel and Alloys by Krupin in 1965. This mill was a two-high reversing mill with its frame located within the vacuum chamber. A vacuum furnace was located on each side of the mill. According to Krupin, a similar unit was operated by Smirnov at the Leningrad Polytechnic Institute. Production vacuum rolling was described by Polukhin in which steel and refractory metal tubes were helically cross-rolled. In addition, vacuum-forging equipment was described by Pevzner in 1967; and an assembly for rolling pipes in inert gas, in which workers wore pneumatic clothes, is referred to by Krupin.
The efforts in the United States toward preventing the oxidation of metals during hot working were directed primarily toward the use of inert gas. The state-of-the-art as of 1956 for fabricating under inert gas is given in Steel. Kolbe, at the General Electric Co., forged molybdenum and tungsten at 2,000° C in inert gas. Semchyshen and Barr, at Climax Molybdenum Co., constructed two different argon-filled enclosures around a press and furnace. The first unit accommodated an operator wearing breathing apparatus, and the second used glove ports allowing the operator to be outside. Neither arrangement maintained sufficient purity of the argon to allow true hot working of the molybdenum ingots.
The most elaborate inert processing facility was the In-Fab room of Universal Cyclops Steel Corp. described in Steel in 1956, Industrial Heating in 1962, and Iron Age in 1958. The 40- by 80- by 25-foot- high facility, constructed in the late 1950’s, included a forging hammer, reversible rolling mill, induction furnace, and graphite furnace capable of 2,200° C as well as a lathe, saw, welder , and hot shears. The workmen were dressed in “space suits” with intake and exhaust lines. Several smaller inert gas chambers were operated by the U.S. Atomic Energy Commission as described by Kelman in 1956. The operations were performed either by remote control or by workers being outside the chamber and operating with glove ports. Also, three rolling mills for radioactive metals were assembled at the University of California for operation under vacuum or inert gas described in Iron Age in 1962.
Rossin and Mueller reported on a small rolling mill, 3 by 5 inches, enclosed with a vacuum chamber at Universal Cyclops Steel Corp. An induction furnace, also in the chamber, permitted heating samples to 1,650° C. In 1962, Universal Cyclops proposed a large scale “Vac-Fab” rolling plant described in Steel, but this was never constructed. Hill reported on a vacuum rolling mill at Republic Steel Corp, that was used to consolidate titanium powder to eliminate the arc-melting step. The system operated at a pressure of 10 -3 torr.
Habermann described an apparatus for forging at temperatures to 1,800° C under argon, Blickensderfer described laboratory equipment for heating and forging under a vacuum better than 1 x 10 -4 torr at 1,800° C.
Why Vacuum Rolling
Throughout the variety of techniques and equipment described, the literature has shown that metals rolled under vacuum usually have properties different from those rolled in air. The vacuum-rolled metals, in addition to having a superior surface finish, usually have greater ductility, improved impact strength, lower ductile-brittle-transition temperature, better corrosion resistance, and greater electrical conductivity. The rolling process itself in vacuum, contrasted with that in air, is typified by a tendency for the metal to adhere to the rolls, greater forward slip of the metal, and requires more energy.
Early investigations of vacuum rolling established that oxidation could be essentially prevented and that metals which cracked during air rolling could be successfully rolled in vacuum. Ivanov hot-rolled Mo, Mo alloys, Ti, Zr, Zr alloys, Cb, and Ta under a vacuum of 1 x 10 -5 torr. Carter reported that tantalum alloys, Ta-10W and F-48, could be rolled at pressures of 10 -3 to 10 -4 torr, and that the columbium alloy D-31 and the molybdenum alloy TZM could be rolled at pressure up to 10 -1 torr. Krupin rolled tungsten and molybdenum successfully in vacuum. They found that 5 x 10 -3 torr was sufficient because no additional benefit was observed when the vacuum was increased to 5 x 10 -6 torr. Krupin and Pavlov reported an increase in ductility and oxidation resistance after vacuum rolling of W, Mo, Rh, Cb, Ta, Zr, V, Cu, Ni, and other alloys compared with rolling in air. Smirnov discovered that beryllium could be rolled successfully at high speed (0.76 m/s) under vacuum of 5 x 10 -6 torr but it cracked when the rolling rates were low (0.1 m/s).
The early interest with vacuum rolling probably can be attributed to improved ductility. The increased ductility of metals rolled under vacuum has been reported by many investigators. It is usually, but not always, accompanied by a decrease in ultimate tensile strength. An increase in impact strength and a reduction in the ductile-brittle-transition temperature normally occur also. The greater ductility of vacuum-rolled materials is attributed to the absence of impurites, uniformity of structure, and elimination of microdefects as summarized by Krupin.
Smirnov rolled Ti, Mo, and Cb at 1 x 10 -3 to 2 x 10 -5 torr and in air. The ductility was 50 to 85 percent greater and the tensile strength was 5 to 15 percent less for the specimens rolled in vacuum. Gurevich compared the effect of rolling Ti, Mo, Cb, and Cr in vacuum, argon, and air.
Based on oxygen and nitrogen analyses, they advised that Ti, Cb, and Cr be hot-rolled under high vacuum of about 10 -5 torr, but molybdenum could be rolled at about 10 -2 torr. In agreement with Smirnov and others, it has been reported that titanium and molybdenum have greater ductility and lower strength when rolled in vacuum than in air. Gurevich and Orzhekhovsky confirmed the preceding and reported a decrease in the ductile-brittle-transition temperature for Cb, Mo, and W rolled under vacuum. Both papers reported that rolling in inert atmosphere produced intermediate effects .
Bykadorov reported that steels hot-rolled in vacuum exhibited approximately 2 to 60 percent decrease in yield point and ultimate tensile strength, and that the latter was more uniform. The lack of oxide eliminated the need for pickling and the lower yield point obviated the need for annealing before drawing.
Nel’zin and Filippov studied titanium alloys and found that hot rolling under vacuum of 1 x 10 -4 to 1 x 10 -5 torr produced material without scale and with increased plasticity. The mechanical strength dropped by 7 to 10 percent while impact strength increased 10 percent. Krupin reported that the ductility of cast chromium rolled in vacuum of 5 x 10 -5 torr was increased threefold. For rhenium, the red-brittleness was avoided at a vacuum of 10 -1 to 10 -2 torr. The elongation of columbium rolled in vacuum of 5 x 10 -5 torr was 18 percent contrasted with only 3 percent when rolled in air. The ductile-brittle-transition temperatures of chromium and tungsten were decreased 100° to 200° C by vacuum rolling. An iron alloy, N-42, had a 64 percent increase in ductility with only a slight decrease in tensile strength. Krupin and Chernyshev summarized the effects of vacuum on the rolling of metals.
Many other effects of vacuum rolling have been reported in the literature. Gurevich and Zubko reported that the carbon content of the surface of vacuum-rolled steel was higher than the same steel rolled in air, which normally has a depletion of surface carbon. The sulfur decreased less by rolling in vacuum than in air. Also, the coefficient of friction of the steel and its resistance to rolling deformation were greater during vacuum rolling, while specific pressure was several times larger in vacuum. Osipov studied specific pressure, coefficient of friction, and forward slip during hot rolling of tungsten in vacuum. Pavlov measured a coefficient of friction of 0.34 to 0.36 during vacuum rolling. Chernyshev showed that the proportion of forward slip of the metal increased for titanium and molybdenum but decreased for steel when rolled in vacuum.
Krupin and Pavlov reported a significant increase in the corrosion resistance to an aggressive medium of a titanium alloy, bronze, and stainless steel rolled in vacuum. An increase in oxidation resistance also was reported. For example, the oxidation rate at 1,200° C of vacuum-rolled columbium decreased to half, and that of vanadium decreased to one-third. Krupin reported similar benefits to tantalum. He also reported a significant improvement in the electrical conductivity of Zr, Cb, Ta, V, and Cu after rolling in a vacuum of 10 -4 to 5 x 10 -6 torr.
Smirnov showed that titanium, after vacuum rolling below 10 -4 torr, had a more uniform grain structure with larger grain size. Also, the fine-grain surface structure normally associated with molybdenum, and attributed to gas contamination, was eliminated by vacuum rolling. The effect of surface contamination on the microstructure of molybdenum and its alloy, TZM, is discussed in Industrial Heating.
Both thermodynamics and kinetics of metal-gas reactions are relevant to vacuum hot-rolling processes. Considering oxide dissociation reactions and the kinetics of oxidation, Krupin and Pavlov established conditions for an oxidation-free treatment at high temperature. Metals such as W, Mo, Re, Ni, Cu, O8, and others, with relatively high dissociation pressures of the oxide, are nonoxidizing at 10 -1 to 10 -2 torr at their respective hot deformation temperatures. Theoretically, Ti, Zr, Cb, Ta, V, and other metals having oxides with extremely low vapor pressure should oxidize at pressure too low for commercial application of vacuum rolling. But in practice, because of the low rate of oxidation at low pressure, 10 -5 torr is generally sufficient to control oxidation during normal process times. A more complete discussion of basic thermodynamics and kinetics in vacuum metallurgy was presented by King. A mass spectrometric analysis of the gases normally encountered in vacuum rolling was made by Anikeev. Bianchi and Mueller found that argon provided more protection to metals during hot working, for a given oxygen pressure, than did vacuum.
The problem of adherence of metals to the rolls during vacuum rolling has been reported by several investigations in the U.S.S.R. Krupin reported a tendency for tungsten to adhere to the rolls at a temperature of 1,300° C. Osipov experienced difficulties caused by welding of tungsten to the rolls until the rolls were coated with a hard fusible material, which was not identified. Krupin and Pavlov also mentioned the adherence problem with tungsten above 1,450° C. Chernyshev discussed the adherence problem and pointed out that the lack of an oxide film on the metal allowed the bond. Gurevich studied diffusion of rolled metal into the rolls during hot rolling. Chernyshev compared the forward slip during rolling in vacuum with that in air. Pavlov reported on the effects of roll material and surface finish. Polukhin reported that a boron carbide coating on the rolls prevented adhesion.
Bi-metals
Because vacuum can prevent the formation of metal oxide and nitride films during heating and rolling, there is a greater potential for the bonding of bimetal surfaces during vacuum rolling than during air rolling. Dolzhenkov and Krivonosov clad titanium to steels of various carbon content by vacuum rolling. They reported that a temperature of 1,000° to 1,050° C was required to produce the best bond, but a problem with embrittlement of the interface resulted from the formation of titanium carbide. Krupin and Yudina reported on results obtained by rolling composites under vacuum of 1.5 x 10 -5 to 5 x 10 -5 torr. Their composites consisted of commercial titanium bonded to low- and high-carbon steel, stainless steel, and manganese steel. The titanium steel bond was produced in vacuum with 20 to 30 percent reduction whereas in air a reduction of 70 to 80 percent was required. Dolzhenkov studied the effects of vacuum in the range of 10 -2 to 10 -5 torr on the roll bonding of iron-titanium at 1,000° C. They obtained a shear strength of 16 to 22 kg/mm² for specimens rolled at 10 -4 to 10 -5 torr, and 3 to 16 kg/mm² for specimens rolled at 10 -2 to 10 -3 torr. Smirnov bonded large sheets of titanium to steel by enclosing them in a pack which was evacuated during rolling. A shear strength of 15 to 20 kg/mm² was obtained. Amonenko reported on the use of columbium and copper intermediate layers in titanium steel bonds to improve the ductility. Dolzhenkov reported on the vacuum rolling of titanium steel on a pilot scale at a pressure of 5 x 10 -5 torr. The problem of the brittle interface was overcome by using thin layers of nickel-copper at the interface. The resulting shear strength was 30 to 35 kg/mm².
Aleksandrov produced copper-nickel bimetals with bond strengths up to 25 kg/mm² by rolling in vacuum of 10 -4 torr. Rolling in a poorer vacuum of 1 x 10 -1 torr produced bonds with 21 kg/mm² strength whereas rolling in air at 4000 to 800° C produced no bonding.
Krivonosov determined the optimum conditions for bonding of iron and columbium at 1,100° C at 4 x 10 -6 torr. A 10-percent rolling reduction resulted in a shear strength less than 10 kg/mm², but with 30 to 40 percent rolling reduction the shear strength was increased to 30 to 40 kg/mm².
Amonenko reported on the effect of temperature and degree of vacuum on the bonding of steels to copper, titanium, and stainless steel, as well as Cu, Ni, Cb, and Mo bonds to each other. A residual pressure greater than 10 -1 to 10 -2 torr caused low bond strength, even for copper-nickel. Amonenko studied the cladding of high-purity copper-nickel for the electronics industry.
Sadaj reported on rolling steel with Ti, Mo, Al, Cb, Cu, steel, Monel, NiMo alloy, and TZM. Several rolling conditions and temperatures were studied. Subsequent heat treating to promote diffusion was found detrimental to iron-molybdenum bonds because of the formation of Fe7Mo8 or Fe2Mo2C intermetallic compounds.
In the United States, Bonchak and Bianchi patented the method of cladding metals by rolling in argon. Coluitibium-molybdenum and tantalum-tungsten were successfully bonded. Winter in another U.S. patent showed how to roll bond certain materials, such as copper-copper 25 percent nickel and aluminum-stainless steel, in air. His claims required that one component have a recrystallization temperature under 260° C and that the other must be at least 60° C higher.
Advantages and Disadvantages of Using Vacuum
The advantages cited in the literature for rolling under vacuum, especially compared with rolling in air, can be summarized as follows:
- Much less edge cracking and breaking of metal.
- Improved surface finish.
- Elimination of material loss caused by oxidation,
- Improved ductility.
- More uniform yield strength and ultimate strength among samples.
- Improved impact strength.
- Lower ductile-brittle-transition temperature.
- Improved corrosion resistance.
- Improved oxidation resistance.
- Improved electrical conductivity.
- Elimination of pickling step after rolling.
- More uniform grain size.
- Higher coefficient of friction.
- Greater bonding strength of bimetals.
- Positive roll bonding of bimetals not otherwise possible.
- Less rolling reduction required to achieve a bimetal bond.
The disadvantages of vacuum rolling compared with air rolling are:
- Inherent complications and expenses of the vacuum system.
- Inaccessibility to the interior of the chamber.
- Time required for pumpdown.
- Increased rolling power required.
- Increased roll forces (specific pressure) during rolling.
- Tendency for some metals to adhere to the rolls at high temperature.
Although rolling metal in an inert gas system might seem at first to be more practical than rolling in a vacuum system, it is apparent, on review of the literature, that the results have not proved to be as satisfactory.
It is concluded that this is the result of the extreme difficulty in maintaining the required degree of purity in an inert gas to prevent oxidation of reactive metals. For example, only 10 ppm impurity in an inert gas gives a partial pressure of the impurity of 7.6 x 10 -3 torr. In comparison it is not all that difficult or expensive to obtain through use of vacuum pumps a lower partial pressure of impurity gases.
Experimental Procedures
Description of Equipment
Beginning in 1970, Bureau researchers assembled a vacuum rolling mill from available components, and tested some of the concepts reported in the literature. For this purpose a large cubical chamber equipped with many viewports and manholes was available. Evacuation was provided by a mechanical pump of 250 cu ft/min (7 m³/min) displacement. Ultimate vacuum of the system was 3 x 10 -2 torr. The chamber, shown in figure 1, housed a 2- by 4-inch laboratory rolling mill. Access to the interior, which measures 60 by 70 by 66 inches
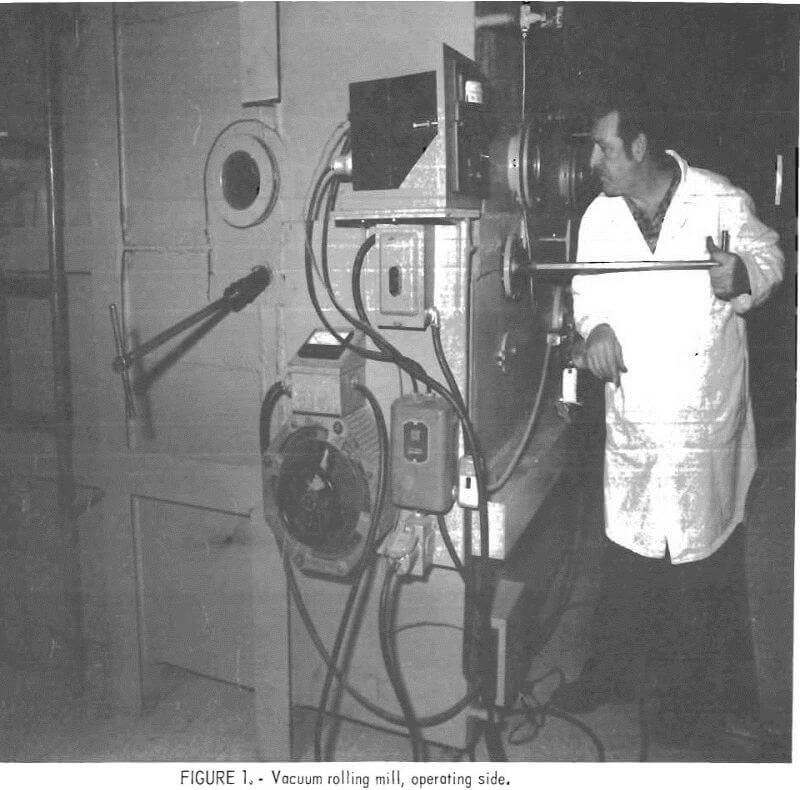
high could readily be made through an 18-inch-diameter manhole located on top of the large chamber. When necessary, the complete rear door shown in figure 2 could be removed. The laboratory rolling mill and specimen furnace are shown in figure 3; also shown in this figure are the two manipulator arms that were used to transfer the samples from the furnace to the rolls.
A direct current (dc) motor, located inside the vacuum chamber, was used to drive the rolls to eliminate the requirement for rotating seals in the chamber wall. The low potential (48 volt) used on the motor prevented electrical arcing that would result at normal alternating current (ac) line potential under low pressure. The dc motor also was advantageous in possessing high torque at low speed. A large storage battery outside the chamber provided the power.
The samples were heated in an insulated ceramic hearth furnace with molybdenum disilicide heating elements. Although this furnace was satisfactory for this system, outgassing became a problem in a lower pressure system.
Mechanical Reduction of Titanium
at Atmospheric Pressure and Moderate Vacuum
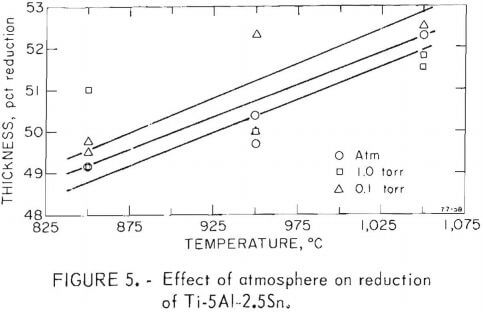
One objective of this project was to determine the effect vacuum rolling had on the mechanical properties of metals. Titanium and two titanium alloys were selected, and were rolled at three pressure levels and three temperatures. The temperatures chosen were 850°, 950°, and 1,050° C for the alloys titanium-6 aluminum-4 vanadium and titanium-5 aluminum-2.5 tin, an alpha, beta, and alpha-beta alloy. Titanium was rolled at 750°, 850°, and 1,000° C. The temperature drop between the furnace and the rolls may have been as much as 100° to 200° C. This problem was kept to a minimum by reducing the transfer time to 10 to 20 seconds; if over 30 seconds, the pass was aborted. The atmosphere of the chamber was controlled at pressure levels of 10 -1 , 1, and 760 torr.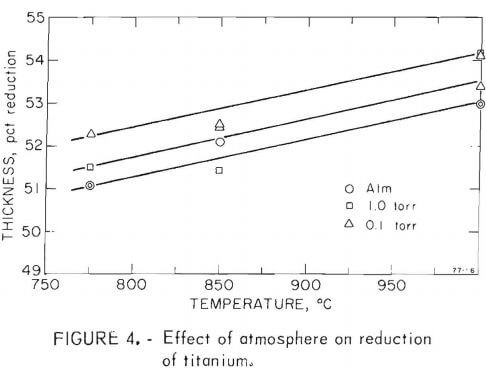
Fifty-four samples were rolled through eight passes under the conditions stated. The percentage reduction is plotted in figures 4-6. All passes were made with the roll separation reduced 10 percent from the previous setting. As expected, there was a consistent change of reduction with temperature. There also was a small but noticeable increase in reduction at lower pressures. Since the temperature dependence is of greater magnitude than the pressure effect, a variation in heat loss due to inconsistent transfer time or due to change in convection could be involved.
 The 54 rolled bars were straightened and machined to dimensions of a standard tensile test specimen of about 0.045 in² cross sections for tensile strength, yield strength, percent elongation, and reduction in area determinations. The results were nonconclusive. The tensile properties of these materials were not affected by air pressure in the range of 10 -1 torr to atmospheric. It is expected that much lower pressures, on the order of 10 -4 to 10 -6 torr, are needed to obtain measurable changes in these properties.
The 54 rolled bars were straightened and machined to dimensions of a standard tensile test specimen of about 0.045 in² cross sections for tensile strength, yield strength, percent elongation, and reduction in area determinations. The results were nonconclusive. The tensile properties of these materials were not affected by air pressure in the range of 10 -1 torr to atmospheric. It is expected that much lower pressures, on the order of 10 -4 to 10 -6 torr, are needed to obtain measurable changes in these properties.
Cladding by Rollins at Moderate Vacuum
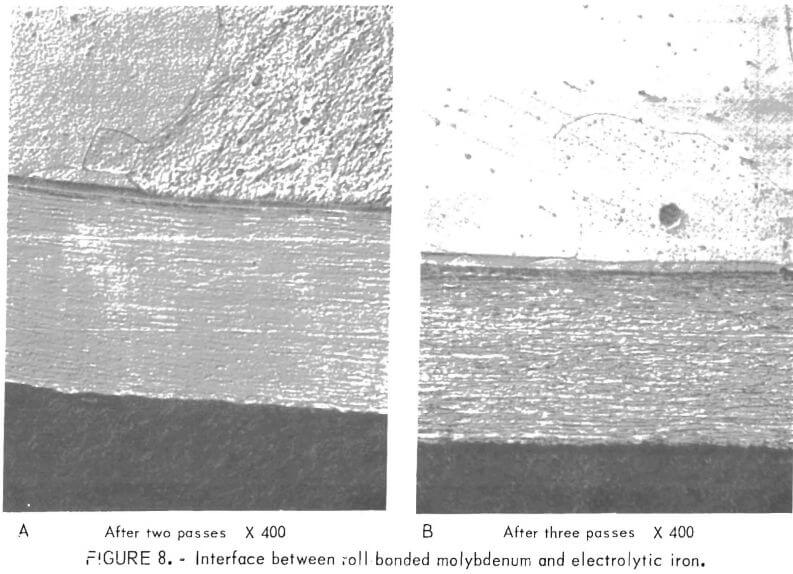
Molybdenum was roll-bonded to both high-purity (99 9 percent) iron and steel Although the specimens were all heated to 1,000° C, it is estimated that there was as much as 100° to 200° C temperature decrease in transfer from the furnace to the rolls.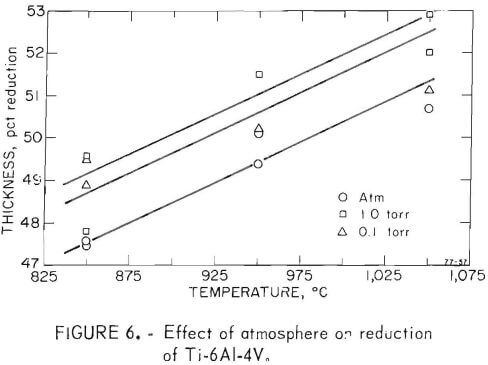 Figures 7-8 show the bond zone between low-carbon steel and molybdenum and between electrolytic (very low-carbon) iron and molybdenum after one and two passes and after two and three passes, respectively. The bond zone became wider with the number of passes made, and wider between electrolytic iron and molybdenum than between steel and molybdenum. Upon examination by electron microprobe, one specimen showed diffusion of molybdenum into iron at a distance
Figures 7-8 show the bond zone between low-carbon steel and molybdenum and between electrolytic (very low-carbon) iron and molybdenum after one and two passes and after two and three passes, respectively. The bond zone became wider with the number of passes made, and wider between electrolytic iron and molybdenum than between steel and molybdenum. Upon examination by electron microprobe, one specimen showed diffusion of molybdenum into iron at a distance

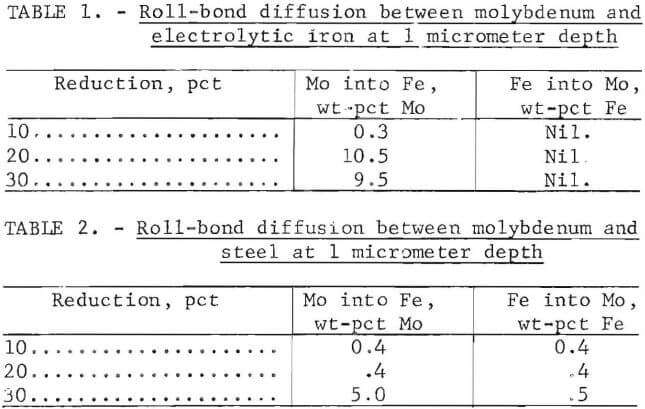
of 10 to 12 micrometers with about 4.2 wt pct molybdenum at the center of the diffusion zone. Molybdenum diffused more readily into the pure iron than into the steel. A comparison is made in tables 1-2.
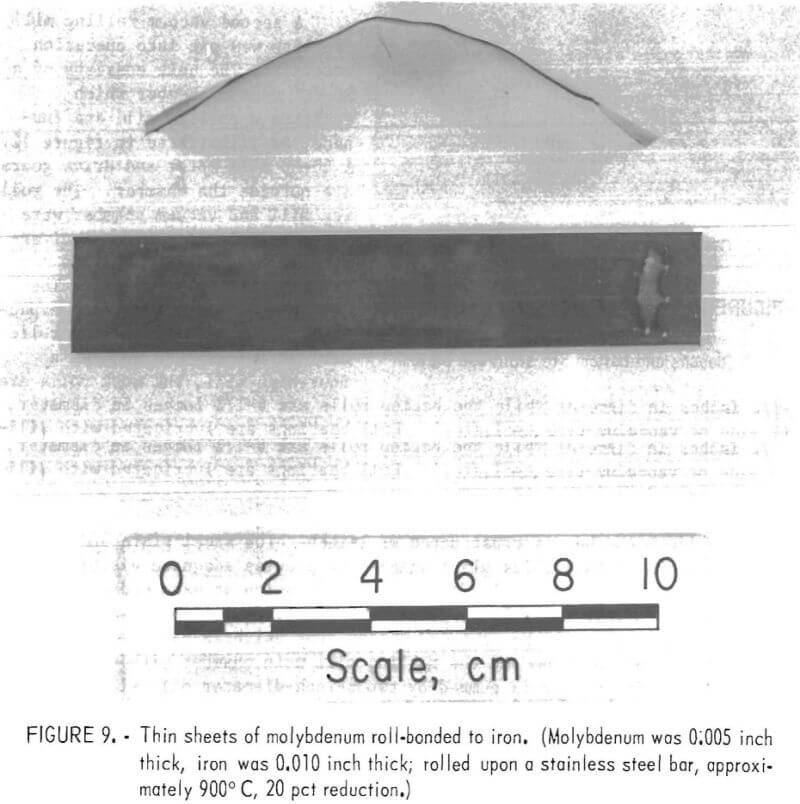
An attempt was made to roll-bond thin molybdenum sheet to thin iron sheet. This was possible when the following procedure was followed: The sheets were cleaned by wire brush and pickled for ½ hour in hydrochloric acid, subsequently rinsed, dried rapidly, and spot-welded together by a small (500-watt) electric spot-welding machine (minimizing surface oxidation). The two sheets, 5-mil molybdenum and 10-mil iron, were further spot-welded at one end to a stainless steel bar (fig. 9). The bar served as a thermal stabilizer to keep the thin sections hot during transfer from the furnace to the rolls. Good bonding of thin sheet was accomplished by heating the pack to 1,000° C and rolling in a chamber pressure of 6 x 10 -2 to 1 x 10 -1 torr.
Bonding molybdenum to stainless steel and iron was accomplished after grinding the surfaces, cleaning with hydrochloric acid, and spot-welding first the electrolytic iron sheet to molybdenum sheet and then tacking them between sheets of 304 and 316 stainless steel. A cross section of the bonded four metals is shown in figure 10.
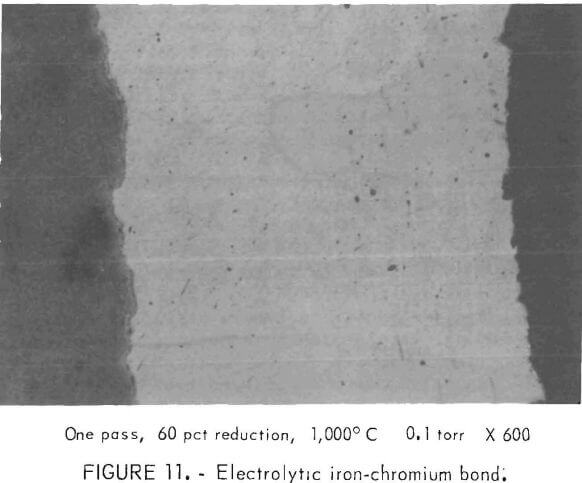
 Commercial-purity electrolytic chromium was hot-rolled from melted buttons to thin sheet at about 10 -1 torr pressure with little difficulty. Roll-bonding chromium to high-purity iron was accomplished, but only after cleaning all surfaces in hydrochloric acid and spot-welding the chromium sheet to the iron. The bond was accomplished by heating to 1,000° C and using a very high reduction (over 60 percent) at 1 x 10 -1 torr pressure. One such bond is shown in figure 11.
Commercial-purity electrolytic chromium was hot-rolled from melted buttons to thin sheet at about 10 -1 torr pressure with little difficulty. Roll-bonding chromium to high-purity iron was accomplished, but only after cleaning all surfaces in hydrochloric acid and spot-welding the chromium sheet to the iron. The bond was accomplished by heating to 1,000° C and using a very high reduction (over 60 percent) at 1 x 10 -1 torr pressure. One such bond is shown in figure 11.
Hot Rolling Equipment
A second vacuum rolling mill facility was put into operation recently. The unit consists of a 50-ft³ vacuum chamber which encloses a rolling mill and furnace as illustrated in figure 12. A 40-hp mill motor and drive gears are outside the chamber. The rolling mill and vacuum chamber were previously located at the University of California, Lawrence Livermore Laboratory. The mill has a separating force capacity of 175,000 pounds. The rolls have an 8-inch face and, as a four-high mill, the work rolls are 1½ inches in diameter while the backup rolls are 6½ inches in diameter, all made of vanadium-type tool steel. Roll bearings are lubricated with silicone grease.
The vacuum chamber is constructed of 1-inch-thick steel plate and includes several 1-inch-thick glass windows to provide adequate visibility of all operations inside. A manipulator in the extension at each end of the chamber allows highly controlled specimen handling. A loading chamber on the entrance side of the mill has a third manipulator which is used to transfer specimens between the room and the rolling mill main chamber without breaking vacuum. The main chamber is pumped by two 6-inch-diameter diffusion pumps backed by a mechanical pump. Typical pressure in the chamber during hot rolling is 2 to 4 x 10 -5 torr. The loading chamber is pumped by a separate 4-inch diffusion pump, cold trap, and mechanical pump.
 The specimen heating furnace, with its low-mass and minimum-surface area, can be heated rapidly with very little outgassing. It is provided with a tantalum split-resistor element of 3-inch-diameter and 10-inch-length inside a quartz tube, and is capable of heating to at least 1,200° C. The quartz tube electrically insulates the heating element from the four radiation shields. At a temperature of 1,000° C, the power in the furnace circuit is approximately 250 amp at 6 v. Doors on the ends of the furnace minimize radiation end losses and are controlled from outside the chamber.
The specimen heating furnace, with its low-mass and minimum-surface area, can be heated rapidly with very little outgassing. It is provided with a tantalum split-resistor element of 3-inch-diameter and 10-inch-length inside a quartz tube, and is capable of heating to at least 1,200° C. The quartz tube electrically insulates the heating element from the four radiation shields. At a temperature of 1,000° C, the power in the furnace circuit is approximately 250 amp at 6 v. Doors on the ends of the furnace minimize radiation end losses and are controlled from outside the chamber.
During rolling, the specimen is transferred to the furnace and rolls while on a specimen carrier, figure 12. By moving the carrier forward, the specimen is brought to the middle, of the furnace where it reaches temperature
in about 8 minutes. Further advance of the carrier transfers the specimen to the rolls. If the specimen does not immediately feed info the rolls, it can be fed by advancing the specimen pusher. Because only a few seconds are required to transfer the specimen from the furnace to the mill, the temperature of the specimen drops very little . Furthermore, the hot specimen carrier helps maintain the specimen temperature. During calibration runs, in which a thermocouple was attached to a specimen, the temperature drop was found to be less than 15° C at 1 ,000° C.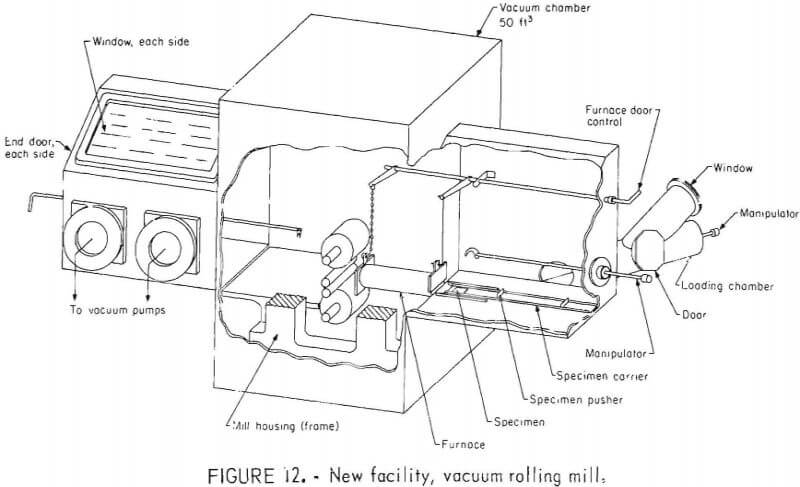
Discussion and Conclusions
From the literature review, it is apparent that considerable attention has been paid to vacuum rolling in the U.S.S.R. It is not certain that the activity has moved beyond the laboratory to the production stage except for tube production. In the United States , despite researchers attempts to gain Government or industrial support for development in the area, most requests have not found support. Vacuum rolling obviously can be practical only when costs by conventional methods are excessive, or when present techniques do not produce the necessary product.
Titanium is a good example of what can be accomplished with vacuum rolling ; it also can be rolled in air. The practice of one major titanium producer is to sheath it in iron, roll, and then strip to obtain the quality required. The extra costs of this operation could lead to consideration of vacuum rolling, even though no property improvement is gained.
Molybdenum also can be rolled in air. But nowhere in the literature has preparation of stainless steel-clad molybdenum been reported. The possibilities of obtaining hot strength with oxidation resistance by this composite should be tempting to design engineers.
Chromium of commercial purity can be rolled in vacuum to form sheet. It further can be bonded to steel in a low-pressure operation.
The commercial utility of vacuum hot rolling of metals only can be conjectured at this time. It will be necessary to obtain further property data to establish whether there are genuine reasons to pursue this difficult but clean process.
