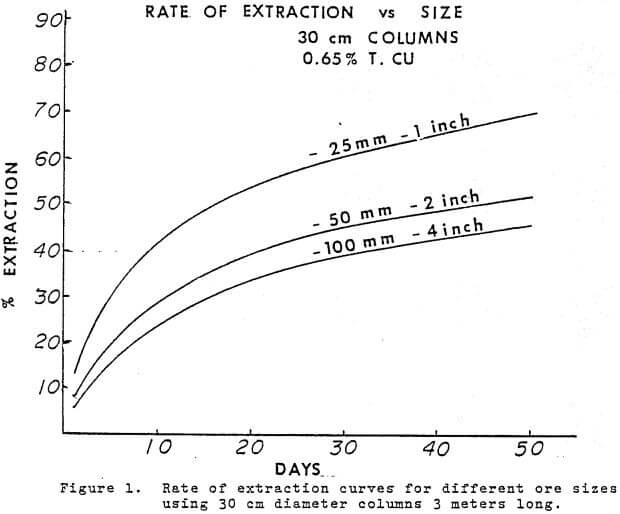
All metallurgical reactions are dependent on time, temperature, atmosphere (reducing or oxidising) and particle size. Particle size has been discussed and atmosphere will be discussed later. Temperature in heap leaching is dependent on ambient conditions and rates of reaction are not greatly effected during most of the year. Freezing in a northern climate or high altitude will impose operating constraints beyond the scope of this paper.
During the operation of the two 50,000 ton experimental test pads, the ambient temperature was below 0° C for several days, however, no adverse effect was measurable on the rate of extraction of copper in the off solutions.
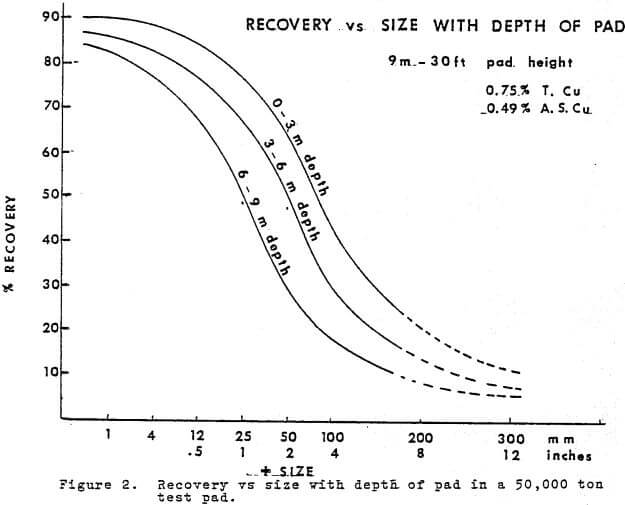
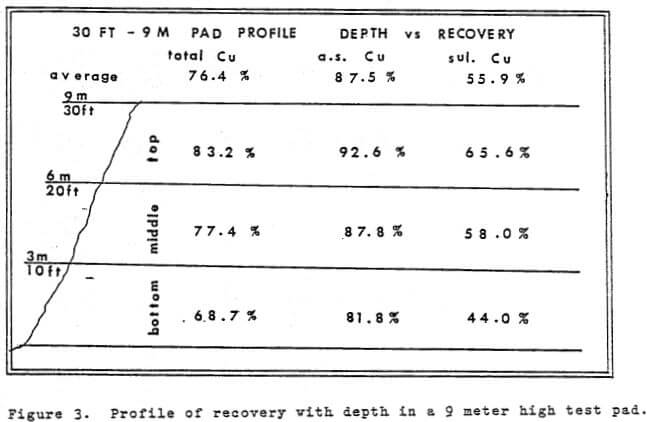
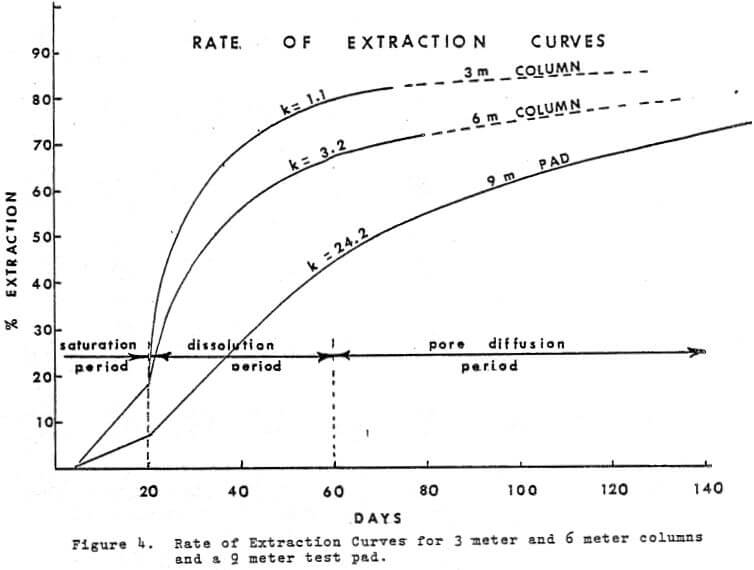
Time is an integral factor in the rate of extraction curve and influences the economics of the heap leaching operation. Figure 4 shows the rate of extraction curves obtained from the 9 meter pad, a 6 meter (20 ft) column and a 3 meter column.
Analysis of the curves based on physiochemical reactions will divide the curves into three parts:
a. Saturation period
b. Dissolution period
c. Pore Diffusion period
a. In the saturation period, the saturation solution should provide the ore with sufficient acid and Ferric ions (in the case of sulfide presence) to contact all copper sites exposed and provide time for reaction. An excess of solution will insure that the lower part of the pad has a solution strength sufficient to attack, the copper minerals. After the application of the saturation solution, a time period of a week, or more will permit sulfation of the oxide type of copper minerals and sulfides to occur. The excess solution may carry out 5 to 15% of the total copper in the pad.
b. When leach solutions (SX raffinate) are applied to the pad after the saturation period, the dissolution period is started and the sulfated copper is ‘rinsed out’ of the ore. The mechanism of dissolution is probably similar to the first day or two in vat leaching or the first hour or two of agitation leaching.
If the ore grade is high (.7 to 1.2% T, Cu) the early solution off the pad will be 30 g/l copper or higher. In 6 meter and 15 meter column tests early “OFF” solutions have been as high as 70 g/l copper.
c. There is no decisive break, between the Dissolution period and the Pore Diffusion period. In the case of Figure 4, the pore diffusion period is assumed to start after 60 days. Pore diffusion per se actually starts during the saturation period however it becomes the dominant source of copper only after the sulfated copper and liberated minerals have been removed during the 1st 60 days. In the case of the 2 meter test pad, Figure 4, the objective was to extract in 60 days and after that time the pad was under pore diffusion control and contributed an additional 16% in the following 60 days.
The curve can be represented mathematically by the formula:
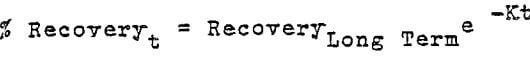
The values of K becomes 1.11, 3.12, and 24.2 for the 3 meter and 6 meter columns, and the 2 meter pad respectively. The significance of the E values is not too well understood and represents a number of variables very difficult to measure.
In the case of the two columns of similar diameter but double in height (K = 1.1 and 3.12) it can be concluded that height is the dominant factor. The 9 meter test pad K value of 24.2 suggests that the total mass rather than the height is the dominant factor. In either case the dissolution period of leaching seems to establish the K value. Further work in this area would be useful in fine tuning our understanding of the process. From the operational point of view, it appears that with the higher the K value, a more uniform rate of extraction occurs during the dissolution period. This will provide a more uniform feed solution to the S-X plant. A small K value will result in wide fluctuations of copper in feed solution to the S-X plant.
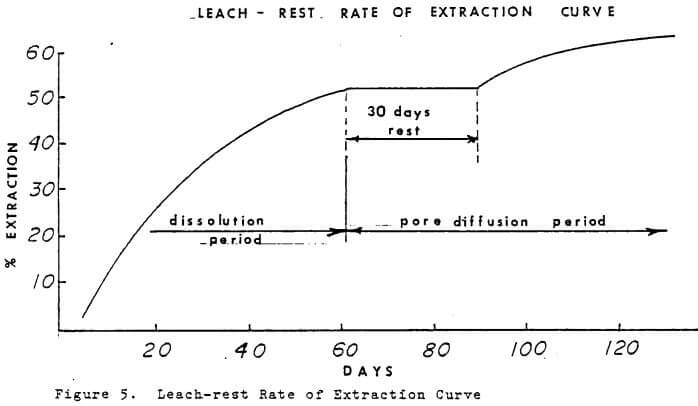
A leach-rest system is used by some dump leaching operations which takes advantage of time. During the rest period if pyrite is present some acid may form. Acid in the residence solution along the the ferric ion will cause dissolution of copper minerals. When leaching is started after the rest period, there will be an increase in the copper in off solutions and the iron will be lower and predominately ferrous.
A test column was operated on a leach-rest system to determine the benefits during the pore diffusion period when grade of copper in off solution was low. The rate of extraction curve is plotted in Figure 5. After a 30 day rest period, the leach solution was started and for a short period the grade increased. After the residence volume was displaced, the grade dropped to the pre-rest period level. The principal virtue of the leach-rest system appears to be solution economy and iron economy if launders are used.
 |
 |
 |
 |
 |
 |
 |
