Table of Contents
The scorification assay for telluride ores has long been believed, and apparently proved, to give low results by reason of volatilization, and it is now seldom used for ores of that class. The assay by crucible is supposed to be far more reliable, and Furman maintains that it is entirely so when properly carried out. He gives a very few comparative determinations by fire and wet assay in proof of his assertion, but the tests are too few in number and the agreement is not so close as might be desired. A more critical investigation of this problem seemed to promise results of some value to those engaged in mining and smelting the telluride ores which are so important in certain parts of our own and other countries, and the data and conclusions set forth in the following pages are the outcome of experiments on telluride ores from Cripple Creek, Colo. From them it will be seen that there is no occasion to question the substantial accuracy of the crucible method, as tested by careful determinations in the wet way. Incidentally the question of losses of gold in assaying, particularly during cupellation and parting, has been looked into, for concerning these points the most contradictory statements are to be found in the literature. Only after most of the work upon the ores had been completed was this fully impressed upon us and the extent of some of these losses duly appreciated. Had this appreciation come earlier we might have been able to offer still more satisfactory results for our assays.
Assay of Cripple Creek Telluride Ores
Nature of the Ores Assayed
Efforts were made to procure two ores of somewhat widely differing gold content and, with a view to the preparation of a homogeneous sample, containing as little free gold as possible. As received, through the kindness of Mr. W. M. Bainbridge, of the El Paso Consolidated Gold Mining Company, and of Dr. Waldemar Lindgren, of the Geological Survey, the samples were marked 6 and 12 ounces per ton respectively, but they were actually much richer and of nearly equal value, approximately 15 and 19 ounces to the ton. At least 10 pounds of each was available. The ores were from a narrow fissure in granite and were themselves practically granite, carrying pyrite in some quantity, the higher grade sample b more than a, some calcite; also traces of copper and molybdenum, as shown by determinations on 100 grains. Other constituents were not looked for. The tellurium also was quantitatively determined, in 50-gram portions, in order to judge of the composition of the telluride mineral. As given below, silver is, no doubt, a trifle low, because of the impossibility of making exact allowance for loss in assaying.
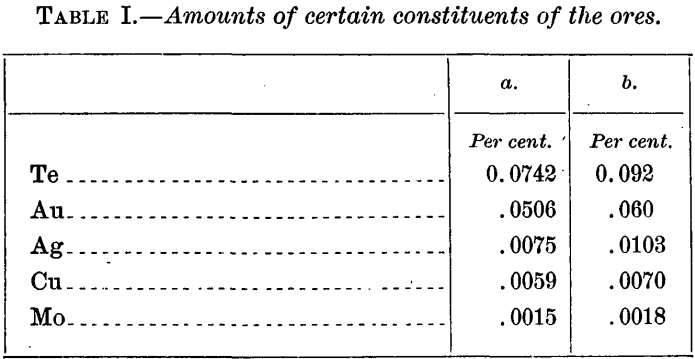
Assuming none of the gold and silver to be free, but all in combination with tellurium, the following percentage compositions for the telluride have been calculated from the foregoing table:
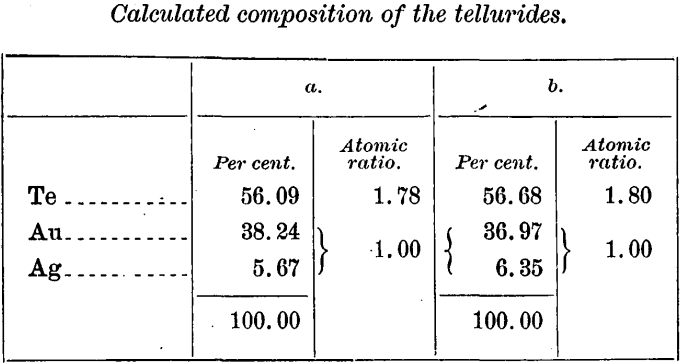
If the tellurium has been correctly determined, the above results indicate the probable presence of free gold, for from what we know of the occurrence of gold-silver tellurides at Cripple Creek they probably all conform to the general formula MTe2. That the telluride here present must then be largely if not wholly sylvanite, assuming absence of other silver minerals, is indicated by the amount of silver shown by the analyses, which is considerably in excess of that in even the richest known calaverite. It is not impossible that both calaverite and sylvanite as well as free gold are present. From the ratio of gold to silver in a second pair of samples, a1 and b1 which, as mentioned later, were taken from a lower position in the original sample bags, so that a considerable mechanical sorting had ensued since the bags were filled, the suspicion may be justified that the silver is in part a constituent of some mineral other than a telluride. For if the assays are correct there is a diminution in the silver as the gold increases, as shown by the following comparison:
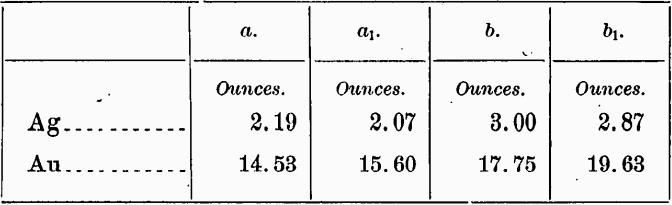
That not all the silver may thus be assigned elsewhere is, however, likely from the fact that the ratio of tellurium to gold alone is much in excess of 2 to 1. But too much weight must not be attached to the above values for silver, and therefore speculations of this sort are of little importance.
Preparation of the Samples
Considerable portions from the tops of the bags containing the coarsely crushed samples received were ground fine enough to pass a sieve of 100 meshes to the linear inch and mixed for what was considered a sufficient time to insure homogeneity. The amounts thus prepared proved inadequate for the completion of the work, so other portions were ground fine enough to pass a sieve of 150 meshes and mixed more intimately even than in the first instance, this being done because the first results on sample b were somewhat less satisfactory and less accordant than those on a, a condition which it was hoped finer grinding would remedy. These second samples were found to be markedly richer in gold than the first, due doubtless to their having been taken from a greater depth in the original bags. They represented practically new ores, and, to simplify references, will be designated a1 and b1.
Combination Wet and Fire Assay
Throughout the literature it is prescribed that for wet assay of gold ores they should be subjected to a preliminary roasting for the removal of sulphur chiefly. Since, however, it is pretty certain that there is loss of gold by volatilization in the roasting of telluride ores, it seemed imperative to disregard these directions and to treat chemically the unroasted ore. No attempts were made to test different methods of attack and subsequent treatment because of lack of time. The method employed in all cases was the following:
Fifty-gram portions in duplicate were mixed with water in large porcelain basins, and strong nitric acid was added by degrees with constant stirring till action had nearly ceased. Then bromine water was added in excess to oxidize the gold and sulphur and precipitate the silver. No artificial heat was employed. The following morning the insoluble matter was collected on filters and washed ten times with water containing bromine and some hydrochloric acid, then two or three times with pure water.
It was found impossible to extract the whole of the gold by wet treatment. The residues always yielded by fire assay small amounts of gold, usually under 0.1 mg. when properly washed, besides nearly all the silver.
The combined filtrates were evaporated in the original porcelain basins to near dryness, then treated with hydrochloric acid and covered for a time. When action ceased the covers were removed and evaporation continued, hydrochloric acid being added two or three times more at proper intervals. When the residues were now digested with dilute hydrochloric acid and a little bromine water, to insure solution of the gold, there remained undissolved considerable calcium sulphate, which was collected on a suitable-sized filter and the filtrate received in a beaker. The first calcium sulphate residues thus obtained were found by fire assay to be free from gold, but all those obtained in subsequent tests were added to and assayed with the main residues.
The filtrates contained all the iron in an oxidized form and a slight excess of bromine. Without attempting to reduce the ferric iron to ferrous by sulphurous acid, whereby tellurium would have been in part precipitated as well as the gold, a large excess of filtered ferrous sulphate solution was added, which induced complete precipitation of the gold alone, without any tellurium. The completeness of precipitation was proved by the failure to detect any gold in the subsequently precipitated tellurium.
After twenty-four hours the precipitated gold was collected on a 9 cm. paper filter of 0.00005 gm. ash content, carefully washed with cold water, then with warm dilute hydrochloric acid to extract iron from the paper, and finally with hot water. Paper and contents were burned in a clean porcelain crucible, and the contents most carefully transferred to the pan of the assay balance and weighed as nearly as might be to hundredths of a milligram. As a check the gold was then generally cupelled with 3 or 4 grams of pure lead in order to eliminate the error introduced by paper ash and any possible failure to wash the filter thoroughly. A companion cupellation with a similar amount of proof gold afforded the correction for gold loss during cupellation. A number of the beads thus obtained from the ore were collectively tested most carefully for silver, with negative results.
It was said above that it was not possible to extract quite all of the gold from the ore, and that the residues insoluble in acid were treated by fire assay in order to recover the amount retained. The retention was probably owing to particles of telluride inclosed in gangue matter, or of gold in sulphur which had been separated but not fully oxidized by the treatment with nitric acid and bromine. These residues were assayed in crucibles with the following charge: Litharge, 2 A. T.; soda, 1 A. T.; borax, 10 grams; argol, 2 grams; salt, cover. The lead button thus obtained weighed about 20 grams and yielded on cupellation nearly all the silver of the ore and a small amount of gold, which latter was obtained pure by direct parting. It was not deemed necessary to examine slags or cupels for their possible gold contents, owing to the minute amount in question, nor was any attempt made to determine by the wet way the exact amount of silver that the ore held.
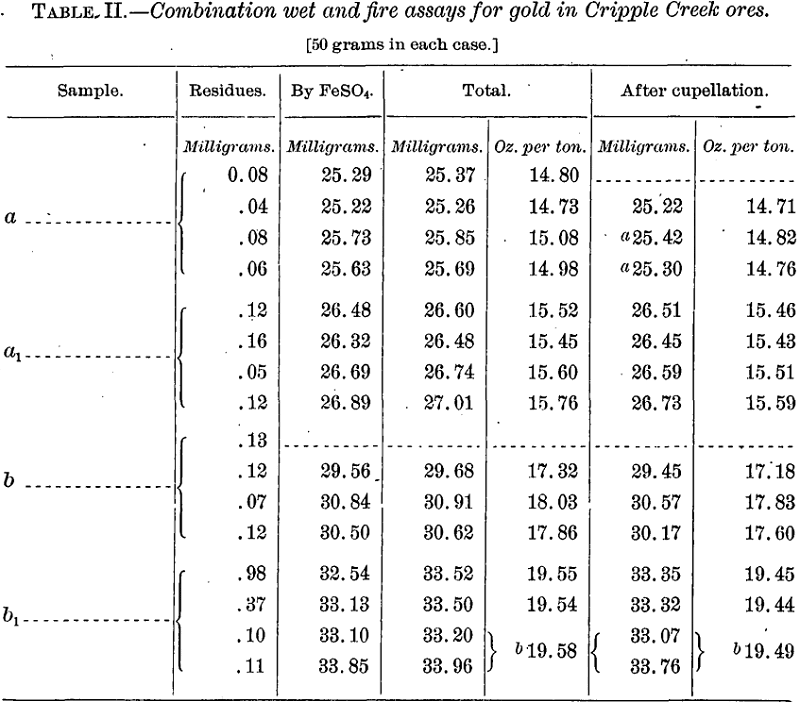
There is seen to be a very fair agreement in each of the several series except b, an exception which was apparent also in the fire-assay tests of the ore. It was largely for this reason that fresh samples of each ore were prepared (a1 and b1) as before mentioned.
Fire Assay
Conduct of the Assay
The assays were all made in Battersea F crucibles, heated in a gas-fed melting furnace, as the muffle available was far too small to admit crucibles of such size. A very satisfactory charge was found to be that given below, and it was adhered to throughout except in a few cases where the litharge was reduced to four assay tons without any apparent difference in results.
Ore……………………………………………………………………1 assay ton (29.166 grams)
Sodium bicarbonate…………………………………………………………….1 assay ton
Litharge………………………………………………………………………………6 assay tons
Borax (fused)………………………………………………………………………….10 grams
Salt cover
Neither iron nor niter was used. In most cases the requisite amount of silver was added to give two and one-half to three times the weight of the gold. During the earlier stages of firing the temperature was purposely kept low, and it was not made high until escape of gas had nearly ceased. The buttons obtained were rather large for cupellation—about 25 grams in weight—but it was felt that by reducing a large amount of lead the collection of all the gold would be more certain. The buttons were in all cases soft and malleable. Not every slag was assayed, since it was found that the gold losses here were very slight, in direct disagreement with Fulton’s results on Cripple Creek tellurides.
The larger number of tests were made by one of us (A), but in order to have the check of another worker a number were made by his colleague (H). The latter’s results seem to be in general a little below those of A, a fact probably explained by the employment of a somewhat higher temperature in cupellation. The powerful influence of slight changes in temperature on the cupellation results will be emphasized in the second part of this paper.
Charges Used in Assaying Slags and Cupels
The charge used for the slag assay was: Litharge, 1 A. T.; argol, 2 grams; salt. For cupels it was: Litharge, 2 A. T.; soda, 1 A. T.; . borax glass, 1.5 A. T.; argol, 2 grams; salt.
Assays for Gold and Silver Together
The first assays were made in order to ascertain not only the gold, but also the silver content of the ores, though no importance was attached to the determination of the latter. Incidentally these tests showed the losses of gold in slags and cupels.
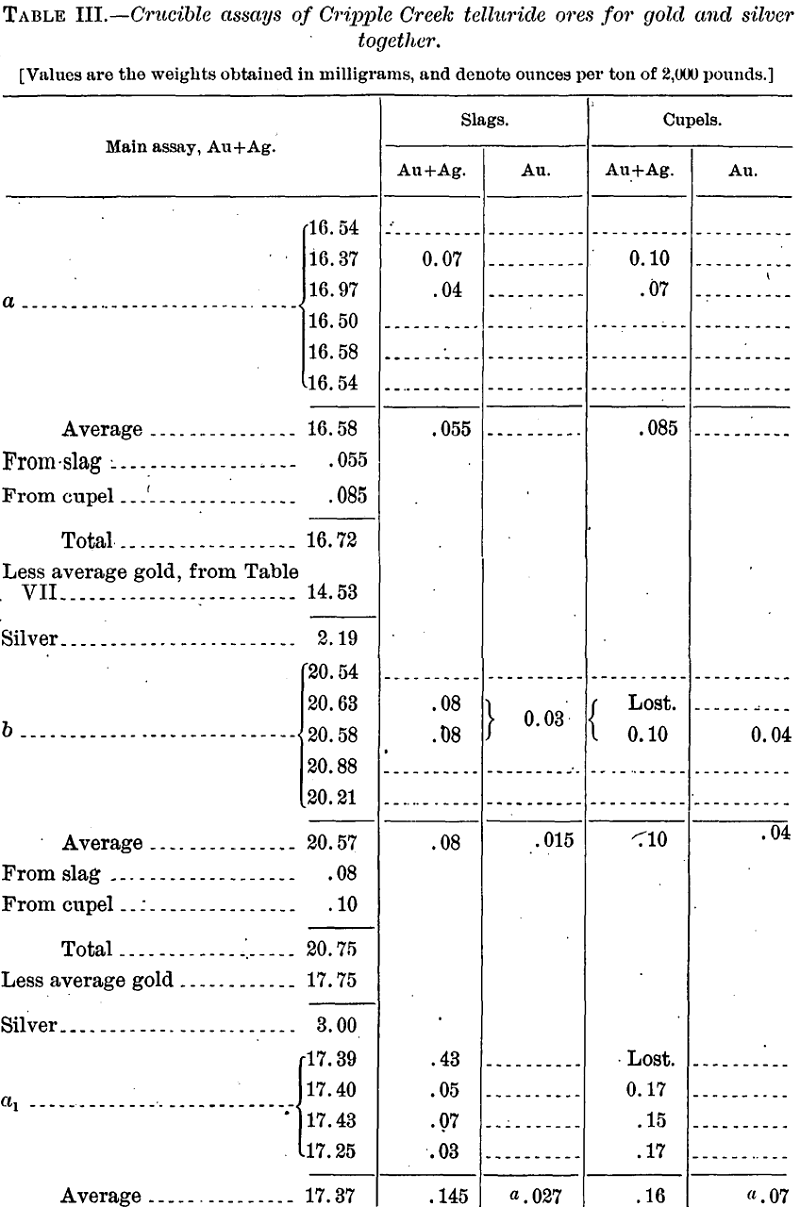
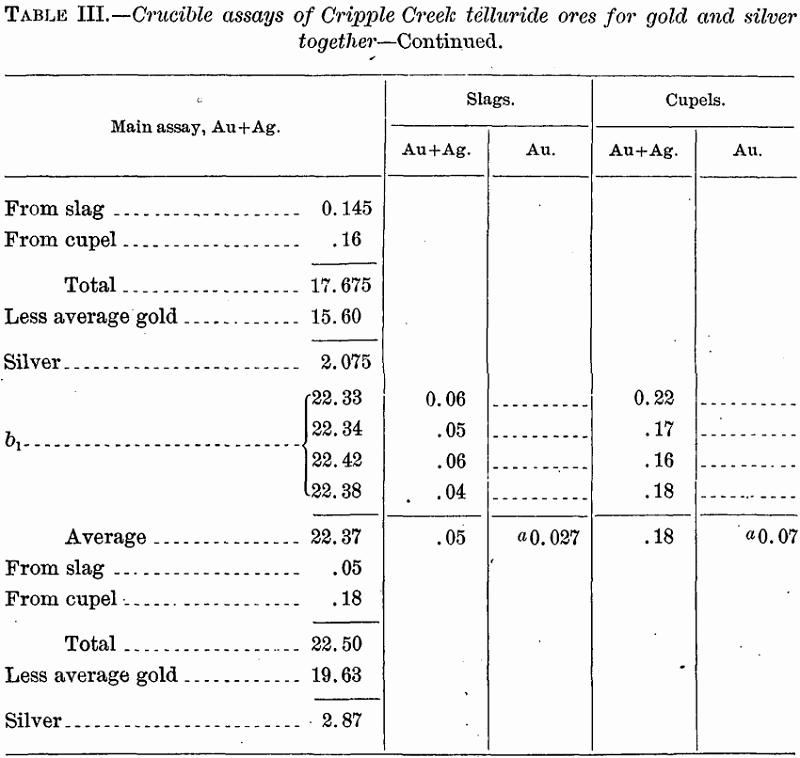
Discussion of results.—The cupellation loss, even without that due to volatilization, is here seen to be markedly greater than in the slag. This observation is in agreement with the general tenor of statements
found in the literature, but directly contradicts Fulton’s statement, already referred to, as to telluride ores from Cripple Creek. May not his high losses in the slag have resulted from his employment of iron nails, with resultant formation of auriferous sulphide in the slag?
In order to have a check upon the actual loss, including that due to volatilization, proof metal of the composition of that obtained from the ore was cupelled with 25 grams of lead. The results follow:
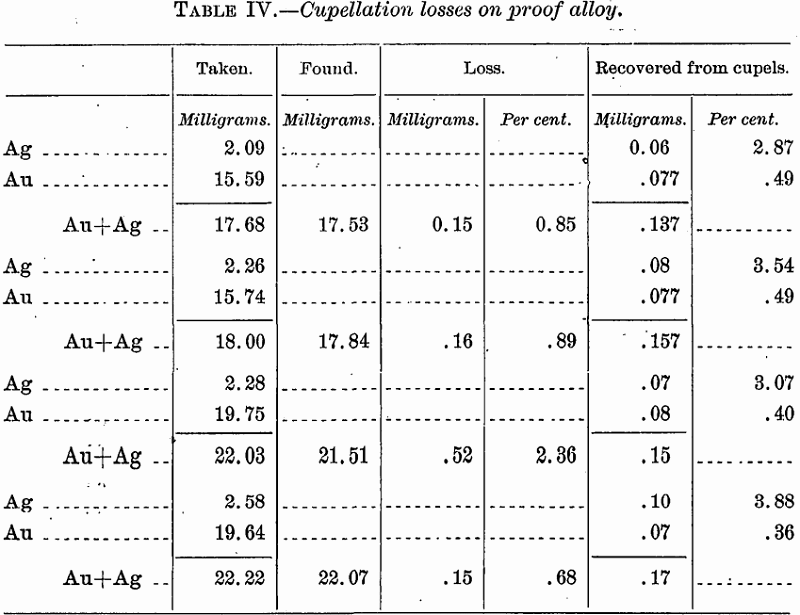
When cupellation takes place at a low temperature, with formation of considerable feather litharge, as in the above cases, the loss by volatilization is seen to be practically negligible, or if not absolutely inconsiderable to be compensated perhaps by retention of lead, but that the case is different when the temperature is higher is plainly shown elsewhere in this paper (Tables VIII and IX).
Assays for Gold Alone
In all these cases the requisite amount of silver was added at the start to give a bead suitable for parting.
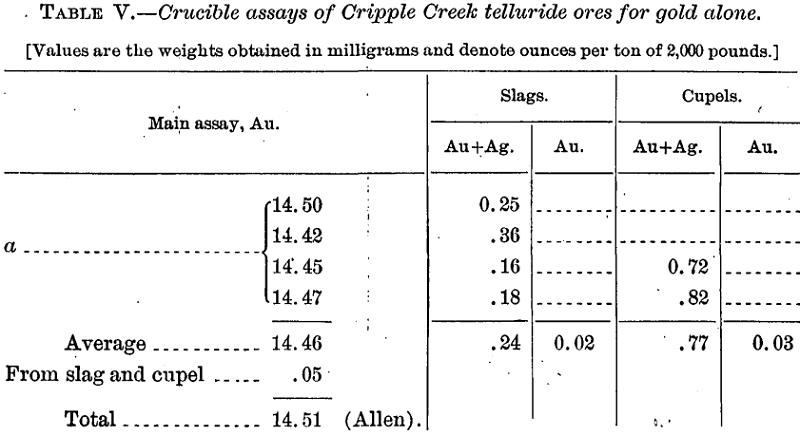
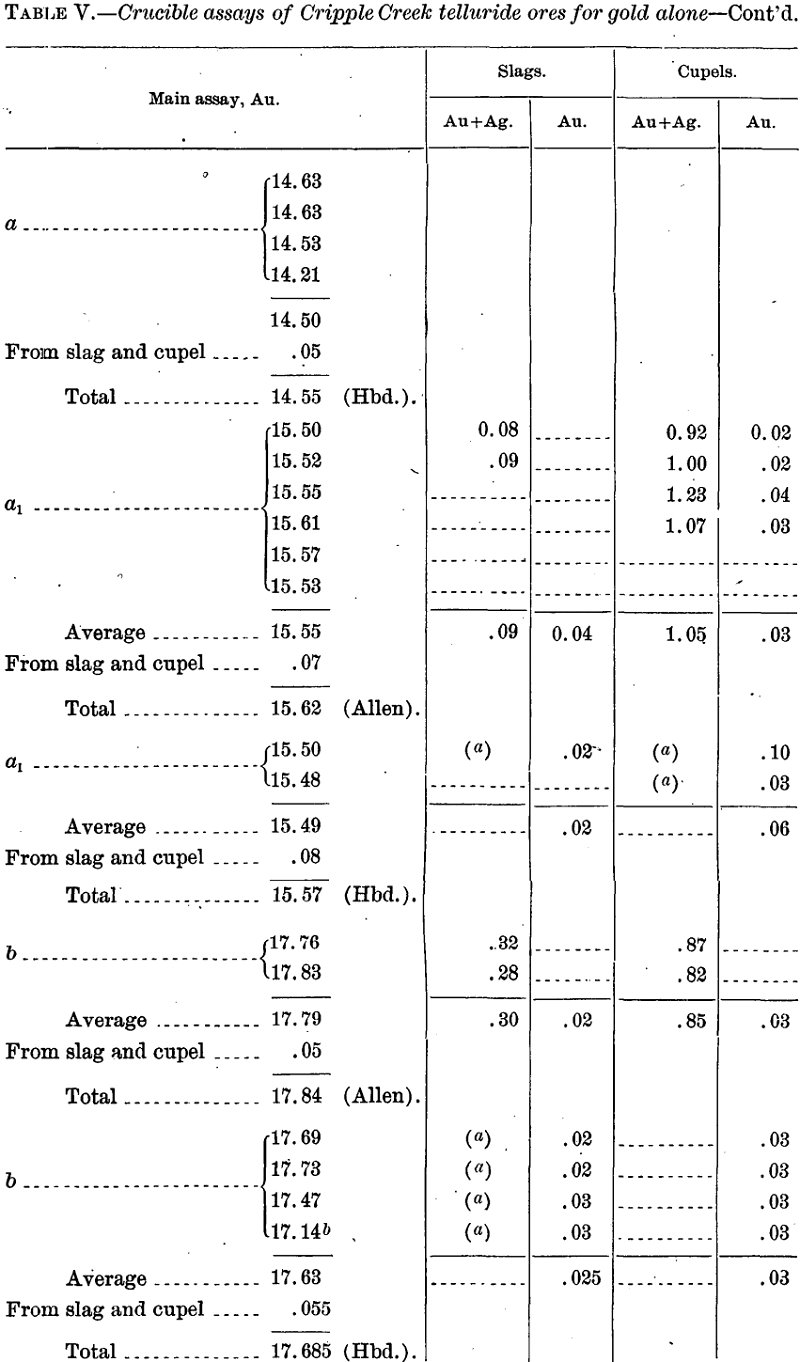
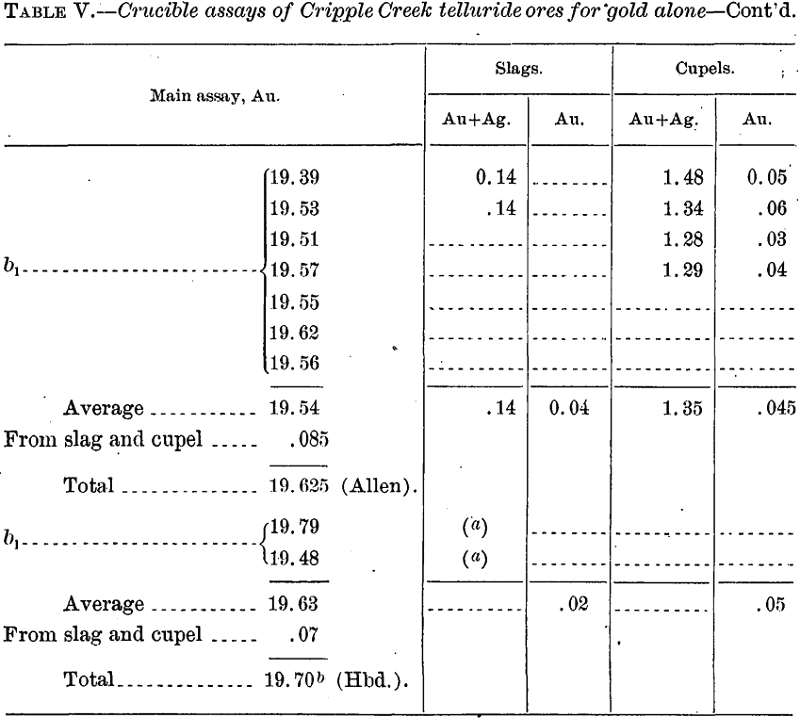
In order to check the actual loss, including that by volatilization, the following cupellations were made with alloys corresponding to the above and about 25 grams in weight:
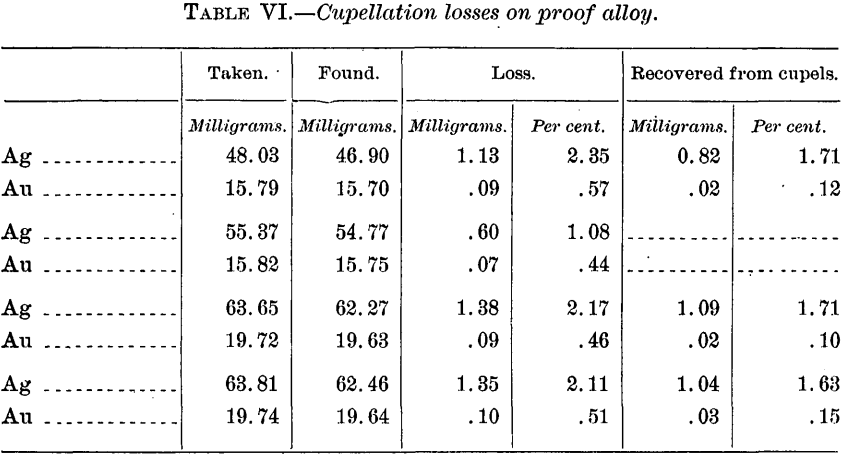
When the above percentage recoveries are compared with corresponding ones for the alloy far richer in gold (p. 15, Table IV), it is seen, as might be expected, not only that there is a selective absorption of the metals, but also that the gold loss by cupellation is absolutely, and, relatively to the silver, greater in the richer gold alloy; but it is singular that whereas in the latter case practically all the lost gold (and silver) seemed to be recovered from the cupel, in the case of the rich silver alloy by far the greater part of the lost gold has been volatilized instead of absorbed. That a portion, and relatively a large portion, is always thus volatilized under similar conditions the results given in Table IX on page 21 clearly show, but there the volatilization loss, no matter at what temperature in the muffle, though very variable, is with one exception considerably below that by absorption. The explanation of this disagreement we are, unfortunately, unable to offer.
Comparison of Results
Comparing the average results by combination wet and fire test and by fire assay alone, we have the following showing:
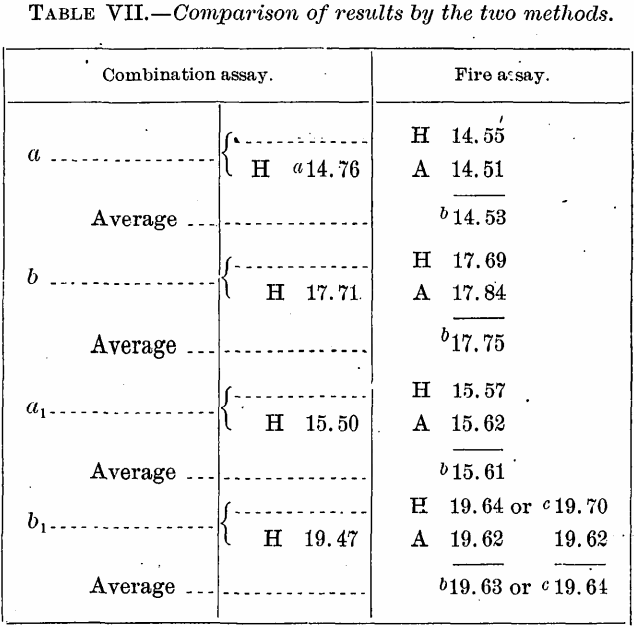
From the above, it would seem to be be clear, as claimed by Furman, that for telluride ores of the composition of those of Cripple Creek the crucible fire assay gives results perfectly satisfactory, as compared with wet extraction. In three of the four ores assayed by us the advantage would seem to be very slightly with the fire assay, but the determinations are too few in number and not sufficiently uniformly in one direction to permit us to regard this as demonstrated.
Because possibly some one may raise the objection that the fire assay failed to extract from the residues insoluble in the liquid reagents the whole of their gold contents, and that therefore the results do not prove the accuracy of the fire assay in comparison with the other, it is to be regretted that recourse had to be taken to the former to complete the wet assays, but there seemed no help for it. No reasonable person will entertain any doubt on the subject.
Part II. The Errors in Gold Assaying
Cupellation Losses
Absorption and Volatilization
That the loss of silver in assaying may be very marked is, of course, well known, particularly that loss which is due to too high heat during cupellation. That gold sulfers similar losses, though to a much less degree, has been fairly well established by various workers. “That loss of gold takes place in cupellation if this is conducted at too high a temperature, or if the alloy is cupelled without quartation silver, is an already long-known experimental fact.” The data relating to gold are, however, not so satisfactory as those with respect to silver, and they are at times uncertain or even conflicting. It is in general said that the cupellation of gold may be carried out at a considerably higher temperature than that of silver, and the assayer is commonly advised to complete the final stage of the operation farther back in the muffle than the earlier stages. Some authorities insist on the absence of feather litharge at the end. There seems to be in the literature no adequate appreciation of the very marked effect that slight differences in temperature actually do produce on the yield of gold by cupellation, especially if the ore be rich, nor of the very considerable volatilization loss that accompanies cupel absorption if the heat is not kept at a minimum. Doubtless these facts are known to individual assayers, but they are not sufficiently emphasized in chemical literature, especially that to which practical assayers in this country usually have access. “ Many yet believe that the degree of heat has little or no influence on the result.”
Our own experiments, which follow, were all made with proof gold and silver from the mint. The results are not therefore necessarily comparable with those obtained by assaying ordinary bullion, where the presence of copper is said to demand a higher temperature and to influence the gold loss.
First, gold alone was cupelled in order to ascertain primarily the loss with increase of temperature, and, secondarily, how this loss was affected by the amount of lead used. Ordinarily six cupels were set in a row, from front to back of the 12 by 6 inch muffle, so that the front cupel always showed at the end abundant feather litharge, and the rear one was at the back end of the muffle. The cupellations were made in most cases with an altogether unnecessarily large amount of lead (25 grams), in order to duplicate as nearly as might be the conditions under which the assays described in Part I were cupelled. Table VIII shows these results.
Then quartation mixtures were cupelled and the silver as well as the gold losses determined (Table IX).
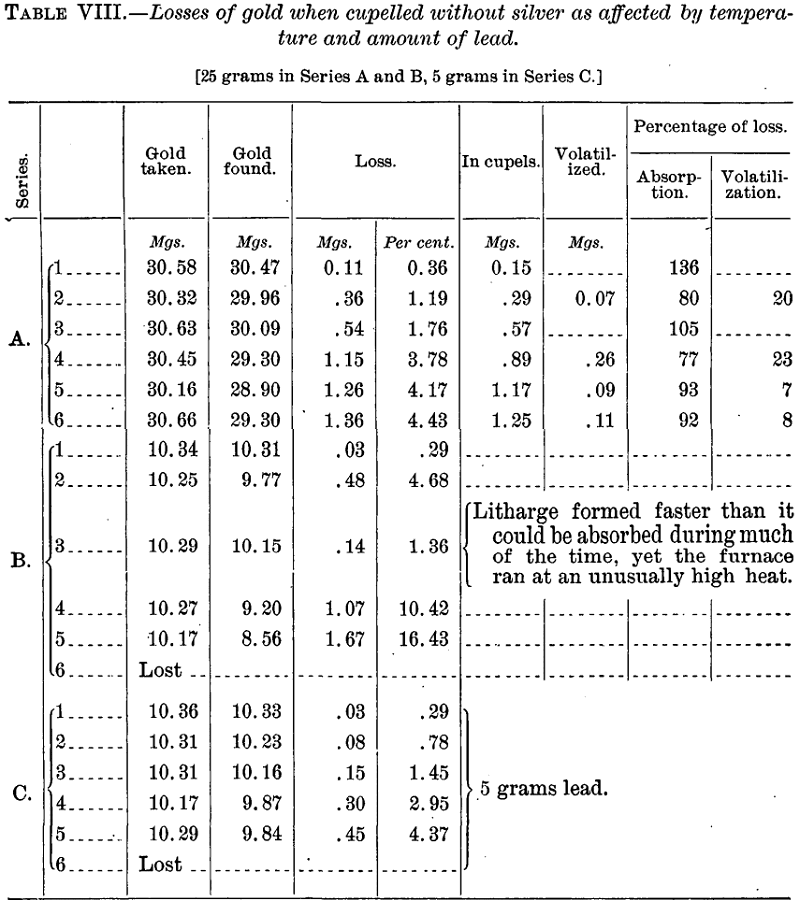
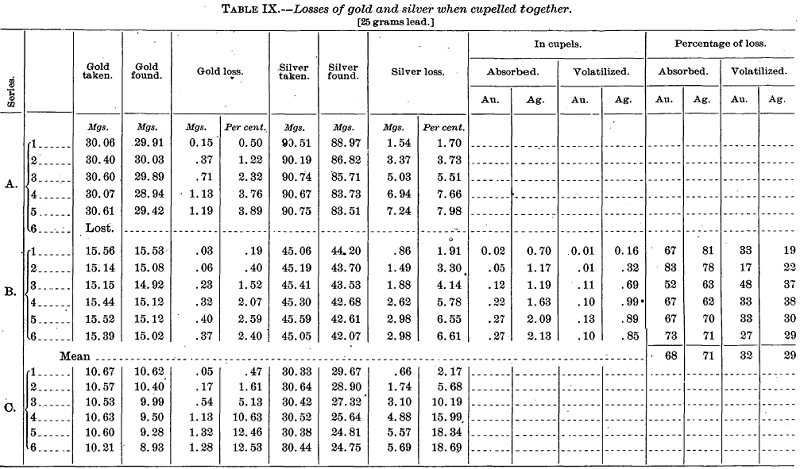
The foregoing tables show most strikingly the effect of increasing temperature on the loss of gold (and silver) and that this loss with pure gold is almost wholly by cupel absorption. A difference of position in the muffle amounting to no more than the width of a cupel is of great moment. The percentages given in the last two columns of Series A, Table VIII, and of Series B, Table IX, are of course subject to considerable probable error because of the difficulty of weighing such small beads to hundredths of a milligram and the probability of losses inherent in the operations by which they were obtained. Further, because of the probable retention of traces of lead by the large gold beads (see p. 24), the volatilization figures are, if anything, too low and those for absorption too high.
It is clear that the following statements are far from correct:
“A high temperature has no ill effect, the loss of gold in cupellation being so small that-it need not be considered. It is about balanced by the silver left in the gold after parting. The cupellation loss is about 0.07 per cent.”
“The loss of gold by cupellation is insignificant. (about 0.7 per 1,000), compensated moreover by the small quantity of silver which the gold retains after parting.”
“All the silver lost is absorbed by the cupel. ”
Our experience goes to show that, as a general direction, the following should be modified, for with pure gold and telluride ores no higher heat is needed than for silver cupellation.
“ A high heat is maintained throughout the operation [cupellation] and especially at the time of brightening, that is to say, a higher degree than is admissible in a silver assay.”
The average loss named in the first two of these extracts is from three to seven times less than the lowest of those in Tables VIII and IX—those suffered at the lowest permissible limit of temperature.
Though not connected with the present discussion, it is impossible to see why the following, credited to Aidarow, should be true:
“The gold, however, only soaks into the cupel when it has been scorified with lead beforehand. If gold is added to lead that is already fused on the cupel, no loss of gold takes place on cupellation.” That a small but appreciable portion of the total loss is due to volatilization the results of Series A, Table VIII, and Series B, Table IX, clearly show. That such loss occurs is mentioned by some writers but most writers make no mention of it. According to Rose 10 per cent of the total loss in bullion assay is by volatilization, a proportion roughly agreeing with the returns in Table VIII, Series A, final columns.
That gold volatilizes slowly if kept above its melting point Napier has shown but this condition does not ordinarily, if at all, obtain after the bead has brightened, so that the direction that it is quite safe to leave gold beads in the muffle after brightening for almost any length of time seemed probably justified. To test the point, however, a number of trials were made by cupelling several beads simultaneously and taking them out at intervals of one-half, five, and twenty minutes after pushing them back in the muffle to brighten. No constant losses were suffered by those left in for the longer periods, but curiously enough there seemed to be a slight tendency to increase in weight. To what this could be attributed is not clear.
On their face the results of Series C, Table VIII, as compared with Series B of the same table, seem to indicate that the amount of lead exerts a very marked effect on the gold loss. Roessler is fully persuaded from his own table of tests that this is generally true. But compare for a moment the three series of Table IX. The same amount of lead was used in them all (25 grams) and the absolute losses of gold with 30 mgs. and with 10 mgs. were practically identical, while with 15 mgs. (Series B) they dropped to about one-third. Here the only seemingly probable explanation is that the temperature throughout the muffle must have been appreciably lower with B than with A and C. Hence it is not improbable that a similar explanation may be the proper one to adopt for the low results of Series C in Table VIII. The cause is certainly not conclusively shown, and it has been made plain that slight differences in temperature produce marked effects.
The final columns in Series B, Table IX, are of interest as showing that with quartation alloys not only is the volatilization loss of gold greater than when pure gold is cupelled, but also that the ratio of volatilization loss to absorption loss is about the same for both gold and silver, or, roughly, as 30 to 70. We regret that similar comparisons were not made for the other series. It is hoped that at some future time opportunity may offer to test this and other points of interest more critically. For the present, however, this is impossible owing to the enforced abandonment of the Survey’s fire-assay laboratory. It is largely due to this latter fact that the work herein described is rather fragmentary and not altogether satisfying.
Retention of Lead by Cupelled Beads
Inasmuch as in hardly a single case did our corrected assays on proof metal show more than was weighed at the start, even at the lowest heat, with full feather litharge, it did not seem necessary to assume the presence of lead in the beads obtained. Nevertheless, in one case a number of them from the same series of assays were dissolved in aqua regia together, the gold precipitated, and the solution evaporated to a few drops. By exercise of the utmost care it was possible to detect a minute quantity of lead, too small, perhaps, to have influenced the results to any appreciable extent.
Later the following experiments were carried out:
Experiment 1. —Three beads of proof gold, weighing, respectively, 30.14, 50.40, and 10.35 mgs., were cupelled with 2 grams of lead each, in the ordinary way, and weighed after boiling with dilute hydrochloric acid to remove any film of lead oxide or particles of litharge-soaked bone ash. They were then dissolved together in aqua regia, evaporated, diluted with sulphurous acid, the solution filtered and evaporated till fumes appeared. The residue, consisting of lead sulphate and a little metallic gold, was transferred to a filter after taking up with alcohol, the sulphate extracted by ammonium acetate, the lead reprecipitated by hydrogen sulphide, reconverted into sulphate, and weighed. The weight was 0.0004 gram, equivalent to 0.3 per cent of lead.
Experiment 2.—Three beads of very nearly the same weights as those first acted on were treated in a similar manner, except that they were left in the muffle twenty minutes after brightening. The amount of lead recovered from all three together was 0.37 per cent.
This shows clearly, as had our earlier experience, that the error, though of some magnitude, is not lessened by leaving the beads in the muffle for a considerable time after brightening. It should be said that the sulphate weighed was certainly of lead and not silver. The percentages above given are, however, to be regarded as approximate only.
Losses of Gold in Slags
From a statement in Fulton’s paper, “Assay on telluride ores,” we were led to expect much greater losses of gold in the slag than appear from the tables in Part I of this bulletin. Fulton says that these are much greater with Cripple Creek telluride ores than with ordinary gold ores. They appear, however, so far as our own work goes, to be slight. We have suggested on page 14 a possible explanation for his higher losses.
Errors in Parting
Strength of Acid
Most diverse is the practice in parting as to the strengths of acid employed. Vauquelin seems to have first used acid of 1.16 and 1.26 specific gravity, and these are the strengths most frequently recommended to-day. But there are not wanting those who prescribe other strengths, or who reverse the order of application—that is, employ the stronger acid first—or who are content with acid of uniform strength.
Incomplete Extraction of Silver
Not less diverse are the statements as to the completeness of the silver extraction. Campredon puts the average silver retention for bullion assay, tested upon an aggregate of 100 cornets, at 0.0002 gram. According to Beringer, the surcharge may amount to 0.05 per cent. In Brown’s Assaying (p. 396) it is said that “A small amount of silver will always remain in the cornet, no matter how carefully the manipulations may have been conducted.” On the other hand, Ricketts and Miller say that the inaccuracy due to silver retention in parting can be avoided by careful work. Later, however, they say (p. 128) with respect to gold bullion, “On the other hand, the gold always contains some silver and occluded gases.” That cornets retain occluded gases was proved by Graham, and Varrentrapp showed that the amount varies with the temperature of annealing.
Our own experience in the present work is that while sometimes by dissolving a number of beads together traces of silver were detected, quite as often none were found. This was before we learned the need for more than two or three boilings with acid. We believe that some of the unfavorable results reported by various writers may have been due to insufficient treatment with acid and inadequate washing. Not infrequently the direction is given to wash three times with water. That this is insufficient for such cornets as are obtained in bullion assay our own tests have proved to us. (See further, p. 27.)
There is great lack of agreement, too, as to the best gold-silver ratio in the quartation alloy. For instance, Chaudet and Kandelhardt are credited in Bodeman-Kerl with the statement that a gold-silver alloy with 2½ parts of silver retains less silver when parted with nitric acid than an alloy with 3 parts. The same authority attributes to Pettenkofer the statement that the separation can succeed well with as little as 1¾ parts of silver to 1 of gold. Rammelsberg is quoted to the effect that pure nitric acid dissolves all the silver out of an alloy containing 80 per cent and more of silver and leaves all the gold behind; and, on the contrary, the separation is imperfect with a proportion of from 15 to 80 per cent of silver.
Impurities in Acid
Of course, if the acid used for parting contains certain impurities, losses will occur. The impurity most to be guarded against is hydrochloric acid or chlorine. Bodeman-Kerl mention also sulphuric and sulphurous acids. Rose ascribes, on the basis of his own tests, 8 per cent of the total loss in a gold assay to the solvent action of the acid, without specifying further. Lenher confirms the statements of several authors as to the solubility of gold in a hot mixture of strong sulphuric and nitric acids, and that on dilution with water it precipitates, this precipitation being supposedly brought about by nitrous acid formed from the nitric acid. He also shows that the sulphuric-nitric acid mixture dissolves gold even at 0° C.
Action of Nitrous Acid
The statement, which, according to Makins, can be traced to Berzelius, is met with in several places that loss of gold ensues in parting from the solvent action of nitrous acid; and Makins, by repeated boilings of assay gold with nitric acid of specific gravity 1.25 and 1.35, was able to establish a steady loss which he attributed to nitrous acid, though the proof is by no means convincing, as he does not clearly show the entire absence of traces of hydrochloric acid in his nitric acid, nor of silver in his cornets, nor did he evaporate the filtrates and prove that they contained gold. The active agent could not be nitrous acid formed from the solution of the quartation silver for its formation must have practically ceased after the second or third boiling. Van Liew obtained astonishing results. He condensed nitrous acid, which he prepared by reducing potassium nitrate with lead and treating the nitrite with sulphuric acid, in a 30 per cent solution of nitric acid in which had been placed leaf gold. At 15° C. about 1 to 2 per cent of the gold was found to have dissolved, and upward of 38 per cent at 82° C., and 51.9 per cent at an undetermined lower temperature.
In order to obtain light on this point one of us prepared nitrous acid and tested its effect on spongy gold. Cold aqueous solutions were obtained by the method of Lunge from starch and nitric acid.
Experiment 1.—Twenty-five c. c. of the solution, containing 0.032 gram HNO2 and 0.401 gram HNO3 per cubic centimeter, was placed in a pressure bottle with very thoroughly washed spongy gold and left in contact for thirty-six hours, the final temperature being about 30°. Evaporation of the filtrate to dryness showed a small, yellowish-white residue, which proved to be mostly organic (a blank test gave the same result). When this was heated to redness a faint purple stain remained. This, when dissolved in a few drops of aqua regia and tested with stannous chloride, showed a trace of gold.
Experiment 2.—The solution was more concentrated, holding 0.0548 gram HNO2 and 0.075 gram. HNO3 per cubic centimeter. A trace of gold was found.
Experiments 3 and 4.—In each of these 10 c. c. of solution, containing 0.062 gram of HNO2 and 0.219 gram of HNO3 per cubic centimeter, was sealed in a tube with spongy gold and heated for seven hours at about 150°. No gold was found in either case.
It is difficult to see how the above conditions can have differed materially from those in Van Liew’s experiments; nevertheless, nitrous acid was made from pure potassium nitrate arid its action on spongy gold observed at a temperature of about 70° to 80° in pressure bottles. No solution of gold could be proved.
In all of the above experiments the acid was filtered through a close plug of ignited asbestos before testing for gold.
The results of these severe tests show that solvent action by nitrous acid during an assay need not be considered. The reported precipitating action of nitrous acid in dilute solution (see above, p. 26) seems to be in direct opposition to such solvent action. Van Liew’s abnormal results must have been due to using a nitrate containing chloride as the source of his nitrous acid.
Action of Nitric Acid
To test the possible effect of nitric acid itself the following experiments were made:
Four portions of pure gold were cupelled with three times their weight of silver and 5 grams of lead each. Five boilings with acid, with intervening washings, were needed to extract the silver to such an extent that an ordinary test failed to show it longer in the extracts. Then No. 1 was boiled with 20 c. c. HNO3 for fifteen minutes, washed, annealed, and weighed. No. 2 was boiled two periods of fifteen minutes each with acid and properly washed, then annealed and weighed. With Nos. 3 and 4 the boiling with acid was repeated two and three times, respectively, the duration of acid treatment in case of No. 4 lasting between one and two hours. The following table shows that no systematic losses appear, and that in the most severe test no loss whatever occurred:
The acid thus used was tested for gold as follows: It was evaporated in clean porcelain dishes, the residues were moistened with a few drops of water and wiped out clean with small pieces of filter paper, these were wrapped in lead cornets to which a tiny piece of silver was added, cupelled, and parted.
No. 1 gave no gold.
No. 2 gave 0.01 mg. gold.
No. 3 gave 0.01 mg. gold.
No. 4 gave no gold.
The acid used for parting was also carefully tested for gold after first precipitating the silver. That from the four first partings combined gave no gold. That from the second gave 0.02 mg. gold in all. This gold may have been float, gold, though the utmost care was taken to guard against this source of error.
From the above it is plain that the losses in parting with pure acid, whether traces of gold really dissolve or not, are negligible in an ore assay at least.
In conclusion it may be said that the gold beads, after the repeated treatments with nitric acid detailed on page 27, were dissolved in aqua regia and the solutions tested for silver with the greatest care, both before removal of the gold and afterwards. In not a single case could the slightest trace of silver be detected.
Summary of Results
Not all of the conclusions here summarized are to be regarded as fully established, while some are but confirmations of earlier statements by others. More work is needed along certain lines, for there is more than one obscure point connected with the fire assay that needs greater attention than we were able to give it. To what extent the results for cupel losses by different workers will be influenced by a difference in the quality of the cupels used there is, unfortunately, no means of determining. The possibility of such a disturbing influence must always be borne in mind.
It is clearly established that the fire assay by crucible for gold telluride ores gives results which are quite equal to those obtained by the wet way, provided due corrections are made for slag and cupel losses.
The gold loss in the slag is very small and generally negligible with the charge used by us, but the cupel losses are very appreciable, and considering the far greater value of gold than of silver weight for weight, it may be doubted whether the statement that “ The loss of gold in the assay of any ore, except when extremely rich, is too small to require correction” is justified when very exact work is called for. Much depends on what is meant by “ extremely rich.” Doubtless the ores treated of in Part I fall within this category, but the losses are there so detectable that correction seems called for with ores far less rich. In view of the very appreciable errors that always occur and which it is quite impossible to wholly allow for in any ordinary assay, it seems that those who demand an assay balance weighing to less than the hundredth of a milligram are decidedly straining at a gnat if they are willing to swallow the camel of an uncorrected assay.
The cupellation loss of gold by volatilization is generally small as compared with that by absorption, and at a temperature allowing the formation of abundant feather litharge is negligible, or perhaps compensated by retention of lead (Table IV), but the case is otherwise at high temperatures (Tables VIII and IX), when it may average one-half of that by absorption in the case of a quartation alloy. The results of Table VI seem to contradict the first statement, for they show a much greater apparent loss by volatilization than by absorption.
The loss of gold by absorption is a very important one, and is influenced, far more than is generally supposed, by slight changes in temperature. It is greater with pure gold and alloys poor in silver than with alloys rich in silver.
From a limited number of tests it appears that the ratio of volatilization loss to absorption loss for quartation alloys at higher than the normal temperature of cupellation is about the same for both gold and silver, or roughly, 30 to 70. This point needs further testing.
Our experiments failed absolutely to show the need for a higher temperature at the end of cupellation with gold beads than with those of silver. The most exact results were obtained when feather litharge was still abundant at the time of brightening.
Furthermore, it is altogether unnecessary to leave gold beads in the muffle for some time after brightening in order to remove the last of the lead, for there is no loss in weight from so doing, but if anything a very slight tendency to increase.
Our results on absorption as influenced by the amount of lead used in cupellation are inconclusive.
The error caused by retention of lead in the beads is one of some magnitude if the results of two careful tests are to be depended on, which showed 0.30 and 0.37 per cent, respectively, of lead. The amount of this retention is not lessened by leaving the beads in the muffle for some time after brightening.
Silver can be completely extracted from quartation alloys by nitric acid, but more than two repetitions of the acid treatment and subsequent washing are called for if any certainty of complete extraction is to be expected.
Tests made with mixtures of pure nitrous and nitric acids show that the solvent action of the former acid is so slight, if indeed there is any at all, that it need not be considered as a possible disturbing factor in parting.
Similarly it was shown that the losses in parting with pure nitric acid, whether traces of gold really dissolve or not, are quite negligible, in an ore assay, at least.
