Table of Contents
In the cyanide process, gold and silver are dissolved from crushed ore as double alkali-metal cyanides, from which they may be precipitated by such positive metals as sodium (amalgam), aluminum, or zinc, or by electrolysis. Two extreme conditions may be noted. Some works, especially slime plants practising decantation, use a relatively large volume of solution—possibly 4 or 5 tons per ton of ore—nearly all of which may require precipitation, so that the solutions handled are of much lower value per ton than the ore. On the other hand, in some leaching plants it is possible to extract with very little solution, and to percolate some of this more than once through the charge before precipitation, so that the solution to be precipitated may be much less than half the weight of ore, and proportionally richer. Solution intended for further use need not have all its precious metal removed, but any that has to be thrown away should be impoverished as far as is economically possible.
In spite of certain advantages possessed by other precipitants, zinc in some form has been almost universally used. In some of the first attempts to utilize cyanides as gold solvents, a “piece or plate of zinc” was suggested as a precipitant, but extension of surface was early recognized as a desideratum.
Macarthur and the Forrests adopted a “metallurgical filter” of zinc shaving, turned from disks or rolled sheets. They had previously experimented on other forms of zinc, and Macarthur records having tried zinc dust, which had of course been long known as a general reducing agent. It had also been known and used as a precipitant of precious metal from plating and photographic solutions, and comminuted zinc had been patented for recovering copper, etc., from ore leaches. Other inventors proposed the virtual making of zinc powder by the attrition of balls of zinc, and by similar means, during the passage of a stream of gold-bearing solution.
Sulman claimed the use of zinc dust, or fume, in a special apparatus, effecting a more or less regular feed of dust and solution by means of intermittent siphons; the mixture or “emulsion” (as it is still called) rising with diminishing velocity through an inverted cone in which it deposited most of its burden, and being clarified by passing a baffle-box and finally a cloth filter. This system was used at Deloro, Ont.
The first cyanide plants in the United States to use zinc dust were the Mercur (Utah), treating coarsely crushed oxidized or roasted ore, the Drumlummon (Marysville, Mont.) and Delamar (Idaho) tailing plants, followed by the Homestake Sand Plant No. 1 (South Dakota). All these treated large tonnages and used filter-presses to catch the precipitate; the first three, and the Homestake when first installed, used square presses of the Johnson type, but differing in size and design. Nearly all the larger cyanide installations on the American continent now precipitate with zinc dust, and the Merrill triangular press has almost entirely superseded other forms for this purpose. Zinc shavings are still used almost exclusively in South Africa, and in the smaller plants elsewhere. Aluminum dust is used to a limited extent, chiefly on Canadian silver ores.
Zinc Dust Precipitation
The early method of applying zinc dust was to fill a vat with pregnant solution, agitate, add the dust and pump through the filter press. The zinc was first introduced by stirring the body of solution mechanically and sprinkling the dust on the surface. A variation was the use of compressed air at the vat bottom, through a small central cross or coil of perforated pipe. In a few seconds this set the solution in violent motion, and the zinc dust was then scattered on it with a shovel. This was at best a dusty and disagreeable job, and the introduction of oxygen at the precipitating stage was opposed to chemical theory. Elimination of the air, however, and cautious sifting of the zinc dust over the surface before pumping, showed imperfect precipitation—for instance, some tests gave only 75 per cent, of the gold precipitated as against 96 per cent, with thorough agitation by air.
To obviate the use of dry dust, various attempts were made to add it in the form of an “emulsion” or suspension in water or in cyanide solution.
An inverted 3-ft. iron cone was fitted with a short valved hose at the apex; the base was covered with a plate having a covered handhole, two valved inlets for compressed air, one for solution, and a relief valve. This pressure cone was two-thirds filled with solution, followed by zinc dust (say 40 lb. for a 200-ton tank); compressed air was then admitted by an inlet leading nearly to the apex to stir the mixture while adjusting the cover. The relief valve was then closed and the “emulsion” sprayed through the hose on the surface of the pregnant solution, which had meanwhile been stirred with air in the usual way.
At one plant such precipitate as settled on the tank bottom was allowed to accumulate there, while only the suspended portion went to the filter-press. Two grades of precipitate were thus produced, the settled material being decidedly lower in value and hard in texture.
Elsewhere, when the solution was pumped nearly to the tank bottom, a man entered the vat in rubber boots and swept settled material toward the pump intake, a small amount of fresh solution being used to assist the sweeping out. In spite of this sweeping there was a tendency for hard lime-zinc scale to accumulate on the bottom, and to some extent on the tank staves, so that after 6 months there might be several thousand dollars thus tied up in a pair of large vats, only recoverable by periodical “scaling” in which hammers and chisels were used.
Pumping a large tank to the press might occupy 2 to 6 hr., and one might expect re-solution to take place during this period when the zinc was added all at once at the start. Repeated tests showed that, although the precipitation by mere agitation was far from perfect, very little resolution took place during pumping, but the part played by the zinc accumulated in the press was evidently an important one.
The amounts directly precipitated ranged from 20 to 90 per cent., while after passing the press 92 to 98 per cent, of both precious metals had been removed, but only 1 to 4 per cent, of the copper. Recently the positive removal of oxygen from the entire body of pregnant solution before adding zinc has been carried out on a working scale. Long-continued tests show that, by subjecting the solution to a vacuum during pumping, a considerable economy is effected, both in the amount of zinc dust consumed, and in the acid required for the subsequent refining. Patent has been applied for in connection with this modification of the process.
Turbid and very cold solutions, extremely low in alkalinity and free cyanide, present the most unfavorable conditions for precipitation. The quantity of zinc dust required per ton is not proportional to the precious metal content, depending largely on the amount used in side-reactions.
Continuous Precipitation
In adding zinc dust to a “moving stream,” as it is termed in the Merrill patent, a uniform feed is absolutely essential to secure maximum efficiency, the volume added per ton being often so small that any diminution becomes temporarily fatal to precipitation. Feeders of various forms have been designed and used. One of the first and most satisfactory consists of a slow-moving horizontal belt, on the level surface of which is spread a charge of zinc dust in a layer of uniform width and thickness. The zinc usually falls into a small mixing cone, through which an auxiliary stream of solution passes, carrying the zinc by a small pipe to the pump intake. A slow drip of lead acetate or nitrate, or of strong cyanide solution, may be added here to facilitate precipitation. Many ingenious elaborations have been devised to secure uniformity in the fall of zinc and flow of auxiliary solution and chemicals. Formerly the zinc and auxiliary solution in the mixing cone were continuously agitated by a small jet of compressed air. According to data published by Clark a saving of about one-third of the zinc was effected by eliminating this air agitation of the zinc feed, and preventing air from being drawn into the mixing cone.
Another good feeder has a cylindrical roller or pulley slowly revolving at the lower end of a hopper, conveying a narrow ribbon of dust, the thickness of which is controlled by an adjustable slot. Or an auger like horizontal screw may remove the zinc from a similar hopper. All such hopper-fed devices require jarring mechanism to prevent the zinc dust from bridging. A miniature tube mill has been introduced as a mixer to smooth out irregularities in the zinc feed.
Bosqui’s zinc-dust feed system has two relatively small tanks, alternately filled and emptied by means of a tilting launder which actuates a counter and throws a measured charge of zinc to the tank to be filled; the zinc is continuously stirred by a set of jets of solution on a revolving agitator.
Mills has a set of vacuum filter frames submerged in a vat through which the mixture of zinc and solution is circulated by a centrifugal pump, while settling is overcome by revolving rakes.
Filter Pressing
Solution intended to be precipitated and thrown away may be run by gravity to a press at a low point; if for further use, it is generally pumped to a press at a considerable elevation, from which it falls to a storage tank. If the distance between intake and press is considered insufficient to allow of complete reaction, it is increased by leading the pipe in a zigzag line. A zinc press with gravity feed, the clear effluent elevated by a pump to storage, has the advantage over a pump-fed press in that zinc and precipitate are kept out of the Cylinders and valve chambers; the claim is, however, made that the pump feed gives better precipitation with a given proportion of zinc.
A close filtering medium is necessary to retain zinc dust and the extremely fine precipitate obtained from low-grade gold solutions. In early practice the medium was sometimes paper between two cloths, or a single thickness of chain-cloth—a rather expensive fabric. Chain-cloth or cheaper heavy canvas may be covered with a light cheap twill, which is removed and burned at each cleanup to recover adhering precipitate, while the heavy backing is occasionally washed or treated with hydrochloric acid to remove limey accumulations. Another plan is to use two thicknesses of medium-weight cotton twill, the outer being taken off at each cleanup and either washed or burned. The other then becomes the outside cloth and a new or washed cloth is put under it for the next run.
One square foot of net filtering surface for 1.5 tons (say 50 cu. ft.) solution per day, or 6 tons per hour for 100 sq. ft., may be taken as a conservative ratio with clear solutions a press may be run at double this rate for long periods. Colloidal suspended matter soon increases the pressure and reduces the pumping rate.
Two-inch distance-frames are suitable for a press used on gold solutions, 3-in. or 4-in. for silver. When a press is opened, most of the cake readily falls into the wheeled tray placed beneath and the remainder is removed by scrapers. It is normally soft but sometimes caked hard as a result of oxidation or the presence of calcium carbonate; this condition can often be controlled by excluding air from the solution.
It was early found advisable, and is still the custom, when starting up a zinc press after a cleanup, to add to the first charge 50 per cent, or more zinc in excess of the weight normally required; this is gradually diminished in successive charges, until the regular amount is reached. If the zinc feed is cut too low at any time, no effect may be noted until several charges have been thus treated, then the “barren” assays rise suddenly and it becomes necessary to add a considerable excess for several charges, until normal working is restored. Apparently a certain excess of zinc must be maintained in the press, or re-solution takes place there to some extent. This is evident when a press stands idle for several hours; the effluent samples caught during the first few minutes of pumping will be abnormally rich—occasionally richer than the pregnant solution entering the press.
Fineness of Zinc Dust
The virtue of zinc dust as a precipitant is explicable by its fine state of division or, what amounts to the same thing, its extended surface. Waldstein’s patent claimed that the zinc oxide, invariably present, formed a beneficial galvanic couple with the metal, while Sulman’s process involved the preliminary removal of oxide by a solvent. In early practice it was noted that dust containing 1 or 2 per cent, of lead was more effective than purer samples, and this was confirmed by laboratory tests on synthetic alloys. Cadmium seems to have but little effect. That fine division is the main factor is indicated by the fact that the finest unoxidized metallic zinc, made by grinding sifted filings, and levigating in absolute alcohol, can be made even more effective as a precipitant.
To get some idea of working conditions in using zinc dust on the large scale, the particles may be assumed to be equal spheres of a diameter which we may take as 0.0001 in. One pound of this assumed zinc dust will then contain 7544 million particles and will expose 1650 sq. ft. of surface, which is much greater than the surface of an equal weight of shaving. In precipitating gold, the practical minimum of zinc dust is probably between 0.1 and 0.2 lb. per fluid ton of 32 cu. ft., or 50 to 100 parts per million of solution. One-tenth of a pound, uniformly distributed through a ton of solution, would give some 13,650 particles per cubic inch, spaced at an average distance of about one twenty-fourth inch. Doubling the weight of dust per ton reduces the average distance between particles by about 20 per cent. If we could substitute particles of half the diameter, the same weights of zinc dust per ton would give eight times as many particles per cubic inch, at half the distance apart. These considerations make evident the desirability of obtaining zinc dust in a very fine state of division, and the bad effect of stray coarse shot or agglomerated masses. In strong solutions, rich in silver, it is probable that the economic limit of fineness would soon be reached; with gold solutions, it seems unlikely.
Efficiency of Precipitation
Zinc dust is sometimes valued by the percentage of zinc actually in the metallic state, estimated by the reducing effect on ferric sulphate or chromic acid; or the difference between total zinc and zinc as oxide may be taken as metallic. As a guide to its precipitating value this is insufficient, and the fineness, or the speed of reaction, must be considered. As a rule, a good zinc dust will nearly all pass a sieve of 200 meshes to the inch, and in some zinc dusts now obtainable very little over 1 per cent, is retained by a 300-mesh sieve. In either case, the coarser portion is merely accidental material—crystalline aggregates, a few shots, or foreign matter.
A laboratory test based on precipitating effect is carried out by shaking half a gram of the dust with a solution containing an excess of double silver cyanide and a little free alkaline cyanide, and determining the silver precipitated. This is a valuable but not infallible guide in practice. Herz has shown that, to obtain comparable results, extreme care is necessary in adjusting the free alkali and cyanide in the test solution.
A precipitating efficiency of 100 per cent, assumes pure zinc, 1 atom of which should precipitate 2 atoms of silver or its equivalent in gold or copper from the double cyanide. At this rate 1 unit weight of zinc should precipitate 6.03 units of gold, 3.30 of silver, or 1.93 of copper, but in actual practice the results are much lower. Applied to commercial dust, the laboratory test usually shows an efficiency value of 30 to 60 per cent. Some extremely fine and pure “artificial” zinc dusts, prepared by re-distillation of spelter, have shown laboratory efficiencies of 75 per cent, or more, and pure electrolytic zinc powder is claimed to be equally good. Otherwise the efficiency is generally higher when lead is present to the extent of a per cent, or two. Distinctly coarse or granular zinc preparations generally show very low results.
Judged by the actual precious metal precipitated, the working efficiency on a large scale may be a mere fraction of 1 per cent, in the case of low-grade gold solutions. A considerable amount of zinc is always wasted in side-reactions, such as the evolution of hydrogen, reducing dissolved oxygen, or precipitating copper and lead. With rich silver solutions the efficiency may approach 50 per cent., especially if no attempt is made to recover the last traces of silver.
A practical example of mixed precipitation may be taken from the records of the Drumlummon tailing plant, covering three seasons, or 24 months of treatment of sandy tailing by leaching, the silver being largely in excess of the gold.
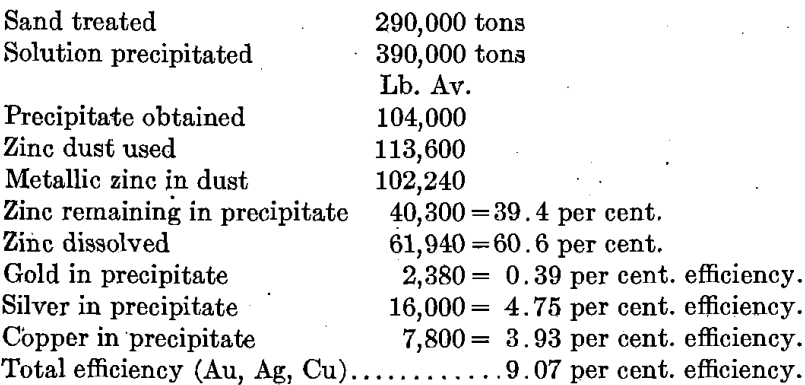
Percentage efficiency is calculated by dividing the weight in pounds by the electrochemical equivalent (Zn 32.7, Au 197.2, Ag 107.88, Cu 63.5, and Pb 103.6, under these conditions) dividing the quotient by the number of equivalents of zinc used, and multiplying by 100. The metallic zinc in dust has been taken throughout at the approximate figure of 90 per cent.
Another instance may be taken from the published results of a month’s run (May, 1911) of Sand Plant No. 1 of the Homestake Mining Co., in which the silver is about 1 per cent, of the gold value. Some lead was added to the solution as nitrate and recovered in the precipitate.
The efficiency for gold was 1.18 per cent., for silver, 0.73 per cent., and for copper 0.19 per cent., in the “weak solution,” making a total efficiency of 2.1 per cent. Similarly the total efficiency (Au, Ag, Cu) in the “low solution” was only 0.71 per cent. At the same time about 1 per cent, of the zinc was consumed in precipitating lead.
That such low efficiencies are tolerated in gold extraction is explained by the fact that, when “low solution” is going to be thrown away, it is obviously worth while to extract the last 2-c. worth of gold recoverable, if this can be done at the expense of 1 c. for zinc, making a fair allowance for refining cost. With zinc at 14.5 c. per pound, 1 oz. Troy costs 1 c. If this ounce is used to precipitate 0.001 oz. of gold (2 c.) from a ton of waste solution the practice is defensible, although the actual chemical efficiency attained is less than 0.02 per cent.
Zinc Dust VS Zinc Shavings
The practical efficiencies obtained with zinc dust have been, generally speaking, about the same as with zinc shavings, and the accumulation of zinc in the solutions is about the same; greater variations occur between two plants using the same process on different ores than between two plants using the different precipitants on similar ores.
The dust process involves a more expensive installation than zinc shavings, but has the advantage of greater compactness and cleanliness, and involves less labor in maintenance and cleaning up as well as less risk of theft. The periodical cleanup is absolute, while a holdover of several thousand dollars’ worth of precious metal commonly occurs with zinc shavings, and makes it impossible to compare the actual with what is often called the “theoretical” recovery. After a destructive fire, precipitate in a filter-press has been found intact, while zinc boxes have entailed great difficulty in the attempts to recover their contents. At a gold plant, a press occupying a floor space of 5 ft. by 14 ft. can easily carry a month’s accumulation of $40,000.
The comparative cost of the two systems at any time depends, of course, upon the wage scale and the relative prices of zinc dust and spelter.
Composition and Treatment of Precipitate
Except in the absence of coarse fibers in the former, there is no essential difference in the composition of zinc-dust precipitate and that obtained with shavings. Both contain metallic gold and silver, usually co-precipitated as an alloy, sometimes amalgamated with mercury or alloyed with copper or with zinc itself—some zinc being apparently re-precipitated electrolytically with the precious metal. Any lead in the solution is also thrown down, while any lead and cadmium in the zinc dust remain with the undissolved zinc, which may form 20 to 70 per cent, of the dry weight of precipitate. Calcium carbonate, moisture, and fine re particles make up the total, with sometimes calcium sulphate or zinc ferrocyanide and basic cyanide. Lead, mercury, and silver may be present either as metals or sulphides, the latter condition resulting from sulphur compounds in the solution. Blowing air through the press to dry the precipitate causes rapid oxidation of zinc, and consequent heating. Dilute sulphuric acid, followed by thorough washing, removes most of the zinc and lime, and more or less copper, while lead, cadmium, mercury and silica remain with the precious metals. The mercury may then be removed by drying and heating in a retort with a little lime. Imperfect washing leaves zinc sulphate, and this, like calcium sulphate or sulphides, yields a matte in the subsequent refining. Hitherto no way has been found to utilize the zinc sulphate solution, impure and generally saturated with calcium sulphate, which is obtained in the acid treatment of precipitate.
Acid treatment, or lead refining and cupellation, or a combination of the two, is necessary before melting low-grade precipitate. Richer gold precipitate, and that from most silver .ores, may be fused with a suitable flux, directly or after roasting, yielding fairly fine bullion.
