Table of Contents
Long ago, zinc refining had not become a general practice among the zinc smelters in the United States. Such refining as had been carried on was confined chiefly to remelting very high-leaded zinc, such as third-draw metal from the retort furnaces, and slab dross, and hard spelter from galvanizing pots. In this remelting operation, the lead and iron were settled out and the refined spelter obtained graded fifth class, i.e., Prime Western. Owing to the presence of lead in most zinc ores, the bulk of the spelter produced by direct smelting is of this Prime Western grade. Careful selection of ores of low lead content gave purer grades of spelter, i.e., Brass Special and Intermediate; and smelters which could secure ores carrying practically no lead produced high-grade spelter. Thus, in general, the grade of spelter produced by any smelter was predetermined by the purity of the ore it could obtain.
The needs of the warring nations of Europe for enormous quantities of cartridge and other high-grade brass resulted in such a demand for the purer grades of spelter that the available supply of lead-free and low-lead ores could not produce the quantity required. Prime Western grades went begging, so to speak, while high grades sold at times from 5 to 15 c. per pound over the Prime Western market. The great demand, coupled with the wide market margin, which permitted the inherent costs and losses, resulted in the conversion of the lower grades into higher grades by processes of refining. These conditions also accentuated the development and size of the electrolytic plants, which were being worked out at the time this increased demand for pure grade of metal occurred. The electrolytic process, where available, produces a higher grade of spelter from impure ores than does the fire process; a large portion of those impurities in the ore, which by the distillation process would be carried into the spelter, are removed in the leaching of the ores and the purification of the solutions.
The refining process, adopted at a number of smelters for the purpose of converting low-grade spelter into grades suitable for brass was that of redistillation.
Impurities in Spelter
The common impurities in spelter are lead, iron, and cadmium; arsenic, antimony, copper, tin, aluminum, etc., very rarely occur. All these impurities are derived from the ores. Of the common impurities, iron is the most generally objectionable for brass; especially for brass which is to be drawn or spun. Lead also must be practically absent, though for brasses that must be machined, a certain amount of lead is necessary to make the cutting clean. For most other purposes, the lead is desired to be as low as feasible. Cadmium up to the extent it occurs in spelter is in most uses of spelter negligible. The processes for refining spelter deal essentially with the removal of iron and lead.
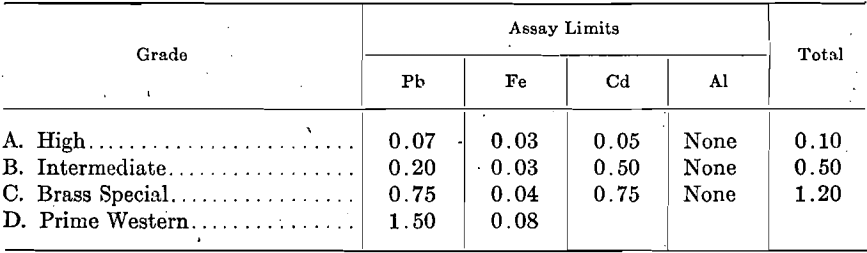
The extent to which these impurities determine the grade of spelter is shown by the following specifications proposed by the American Society for Testing Materials and adopted generally throughout the trade. The Standard Specifications for Virgin Spelter adopted by the Society, Aug. 21, 1911, are:
At the June, 1917, meeting of the Society, the following revision in Specifications for Virgin Spelter was recommended as tentative for consideration, upon which final vote may be taken June, 1918:
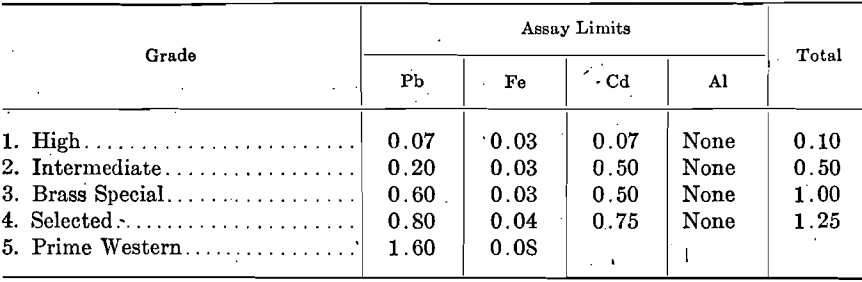
Except the deposits of the New Jersey Zinc Co. at Franklin, N. J., and those of the American Zinc, Lead & Smelting Co. at Mascot, Tenn., and a few other smaller deposits, all zinc concentrates contain from a few tenths per cent, up to 10 per cent, or more lead. The lead content of spelter produced by direct distillation, when treating ores carrying under 1 per cent. lead, is somewhat proportional to the amount of lead in the ores; for example, in order to produce Brass Special spelter, the lead content of the ore is kept under 0.80 per cent.; ores carrying over 1.0 per cent, lead produce approximately the same grade of spelter, i.e., Prime Western. Even when ores contain no more than traces of lead, the readiness with which lead comes over results in spelter containing 0.02 to 0.05 per cent. In consequence, lead-free and low-lead ores command a premium.
Although iron occurs in nearly all zinc concentrates from 1 to 2 per cent, up to 10 or 12 per cent., the percentage carried by the zinc is not proportional to the amount in the ore. The penalty on high-iron ores is due to other reasons.
Cadmium occurs quite extensively in zinc ores, but in small proportions only, usually from 0.20 to 0.30 per cent.; the spelter carries somewhat varying proportions of cadmium. Only first-draw zinc carries a trifle higher proportion of cadmium than the ore, due to the high volatility of the cadmium.
In the distillation process, the effect of temperature and rate of distillation are also important factors which determine the purity of the spelter. The variation in temperature of the retorts from the first to last section and from bottom to top row of the furnace and during the entire distillation period is attended by a variation in the composition of the spelter. The result is very small lots, 300 to 400 lb. of uniform metal; practically every ladleful of metal drawn differs from the succeeding one.
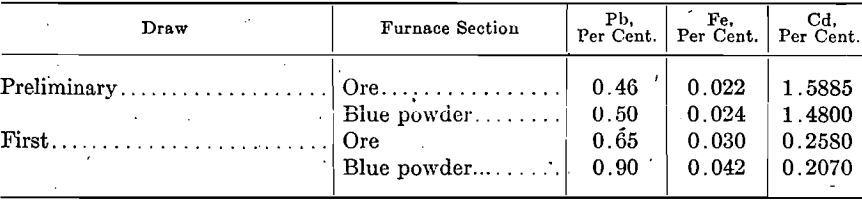
The following table shows the common method of lotting spelter from distilling furnaces, and the assays show the variation in composition of zinc from the same furnace and the same ore:

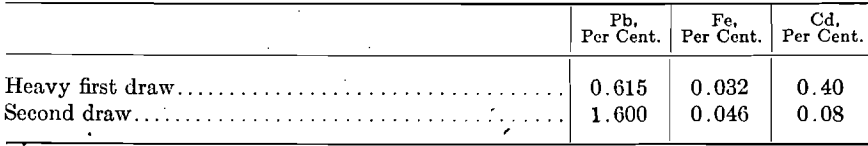
It will be noted that the first-draw metal is lower in lead and iron but higher in cadmium than the other draw metal and that the blue powder metal is higher in lead and iron and lower in cadmium than ore metal. By making a preliminary draw previous to the ordinary first draw, a small amount of metal rich in cadmium, 1 to 2.50 per cent, may be obtained; while the ordinary first draw following will be poorer in cadmium it will be higher in lead, as shown by the following:
Or, by delaying the time of making the first draw, the content of cadmium in the first-draw metal may be reduced, but also at a sacrifice of low-lead content, as for example:
Methods of Preventing Contamination of Spelter
The methods in use for preventing contamination of spelter are at present only relatively successful, but by removing portions of the contaminating elements, they tend to raise the grade of spelter product. In concentrating zinc ores by milling and other methods, the endeavor is made to remove the lead- and iron-bearing minerals, and produce concentrates as free from these impurities as possible. Other processes remove the cadmium by a preliminary treatment of the ore before it is smelted. The electrolytic plants prevent contamination of their spelter through the selective action of certain solvents which do not dissolve the lead, and by means of precipitating agents largely remove the iron and cadmium.
In the distillation process, various means have been proposed but not extensively used, for the prevention of contamination of the condensing zinc vapors. The methods proposed consist of placing dams or filters in the mouth of the retort or butt of the condenser. Filters may consist of a porous pack of suitable-sized refractory material; a porous coal, coke, or charge mass specially prepared, or the last shovel of charge put in the retort may be essentially coke. Dams may be of fire clay or coke. Both dams and filters are effective in reducing the lead and iron content of the zinc, but have no apparent effect on the cadmium. For example, their effect on the lead content is as follows:

Their use has likewise shown the iron content of the zinc to be reduced from 0.07 to 0.02 per cent. Dams, however, have found disfavor, as they tend to reduce the yield of spelter. Part of the zinc condenses back of them in the retort during the early period of firing, and during heavy firing later in the shift this zinc is overheated and largely escapes uncondensed through the nose of the condenser. Filters may lose their porosity through deposition of charge mix, blue powder, or metal, and unless frequently renewed act in the same manner as dams. Preventing contamination of the spelter with iron can be accomplished equally as well by using a scratcher provided with a guard which permits it to enter only to the butt of the condenser. One of the chief sources of iron contamination has been found to be due to scratching a portion of the charge in the mouth of the retort into the molten zinc when drawing metal. A decrease in the lead and iron content of the spelter is also effected by briquetting the entire retort charge. Circular briquets having a diameter slightly smaller than the retort and cored at the center for charging on a rod, yield spelter containing but a few hundredths per cent, lead and a few thousandths per cent. iron. However, such briquets are not practical. It is more feasible to use smaller briquets and charge them in the usual manner, but in this case the amount of lead and iron held back is much less and if the briquets disintegrate during distillation there is practically no change in the spelter composition from that of an ordinary charge. The following assays show the effect on first-draw metal when using small briquets which did not disintegrate:
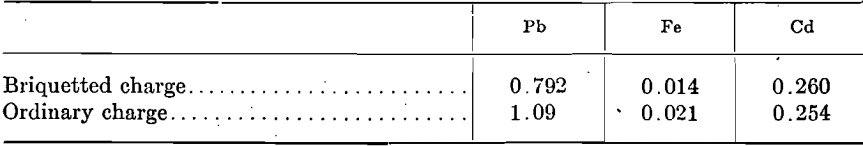
The retention of the lead and iron by the briquetted charge is probably due to the filtering action and the higher heat conductivity of the briquette which produces a more uniform temperature throughout the retort. It will be noted that cadmium, which is reduced and volatilized at a lower temperature than zinc, is apparently unaffected.
Refining Spelter by Redistillation
Although this method of purifying spelter has come into extensive use only during the past 3 years, it has been used in a rough way for a number of years to convert very impure and unsalable zinc, such as third-draw metal, galvanizers’ slab dross, etc., into marketable spelter. Such operations, however, were conducted on a small scale, and chiefly by producers of secondary metals. In 1896, G. M. Holstein and J. D. James took out a patent covering the process of refining zinc and separating it from lead, consisting in redistilling the zinc from impure spelter charged into an inclined retort having the butt end about 4 in. lower than the mouth. The spelter was charged into the retorts in the form of bars. As soon as the spelter melted, the lead, owing to its greater specific gravity (lead 11.4, zinc 6.86), settled out and collected in the lower part of the retort at the butt end. The retorts were carefully maintained at the temperature at which zinc volatilizes, but below that at which lead volatilizes. The furnace for carrying on this operation consisted of a single unit; i.e., the back wall, which in the case of the ore smelting furnace is a center wall and supports the rows of retorts on each side, was an outside wall with openings opposite the butt end of the retort. These openings were for the purpose of cooling the metal in the butt end of the retort, thereby diminishing the ebullition at this point and giving a quiescent condition to the molten metal which promoted the settling out of the lead. By lowering the temperature of the metal in the butt end to below the volatilization point of lead, this chilling also prevented the lead from going over with the zinc. Besides chilling the butt of the retort by exposing it to the air, air might be introduced into the combustion chamber through a hole in the brickwork surrounding the butt of the retort and directly under the retort, or the butt end of the retort might be cooled by means of a water jacket in the furnace wall. An ordinary, conical condenser was temporarily luted into the mouth of the retort for condensing and collecting the zinc distilled over. These condensers were provided with a semicircular bridge wall or dam at their large end, to prevent the boiling metal in the retorts from slopping over into the condenser and also to prevent pure molten zinc in the condensers from flowing back into the retort. The nose of the condenser was partly stuffed.
Two methods are now extensively used for carrying on the redistillation of zinc in a manner similar to the Holstein-James process. The older method makes use of an ordinary smelting furnace block; the later method invented by C. A. H. de Saulles uses a single furnace with back wall exposed.
Converted Smelting Furnace Method
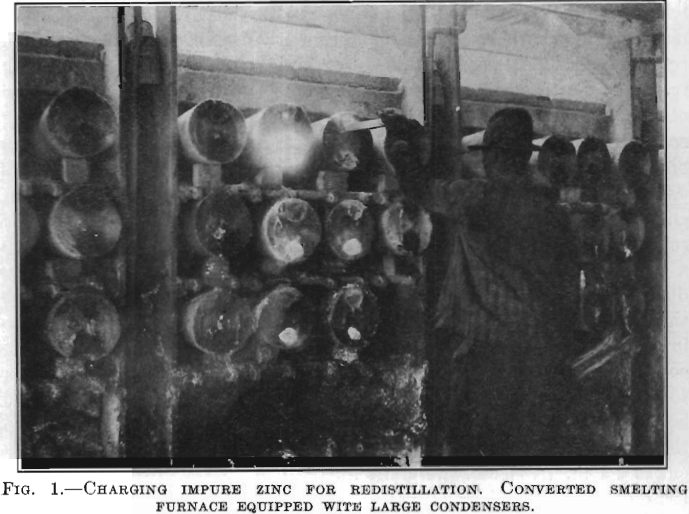
The conversion of an ordinary ore-smelting furnace into a redistilling furnace is accomplished by removing the lower row of retorts and placing the butts of the upper rows on the shelves of the next lower row, which gives the retort an inclination of about 8 to 10 in. in its length and permits it to hold a bath of molten spelter. As a lower temperature is required for redistilling than for smelting, the flue checkers are opened and the combustion gas is burnt under natural draft instead of the common plus pressure condition. This results in a thin flame, more uniform heat, better heat efficiency, and allows the firemen to see the entire retort in the furnace and detect leaks. The size of the distilling furnace, i.e., number of retorts, is obviously predetermined by the size of the original ore-smelting furnace. For example, at one gas smelter the removal of the lower row of retorts reduces the number of retorts per block from 608 to 456; i.e., each furnace is three rows high and has 228 retorts. Some plants use an ordinary condenser which has a semicircular dam in the large end; others use a hand-made condenser which is like a truncated cone, the smaller end (7 in. diameter—17.8 cm.) containing the dam is luted into the retort, while the large outer end (10 in. diameter—25.4 cm.) is closed with a fire-clay plate having semicircular openings at the top and bottom; during distillation these openings are stuffed up in the same manner as the nose of the ordinary condenser. Fig. 1 shows the charging of the zinc into the retort of a natural-gas-fired redistilling furnace with the large condensers. The method of drawing and casting the spelter is similar to the same operations in ordinary practice. The spelter to be refined is either remelted in a settling furnace and then cast, or cast direct from the ore furnace into sticks about 20 in. (50.8 cm.) long by 1½ in. (3.8 cm.) by 1 in. (2.54 cm.) weighing about 10 lb. (4 kg.). Four or five of these sticks make a charge for one retort, the retorts being charged immediately after each drawing. The retorts are charged without removing the condensers, by inserting the sticks through the top opening of the condenser over the dam and giving them a slight push, as shown in Fig. 1. Immediately after charging, the condenser is stuffed and in 5 to 10 min. the chilling effect of the cold sticks introduced into the retort has been overcome and distillation is resumed. The furnace is kept at a uniform heat, no increase or cooling off takes place as with ore smelting. The condensers are drawn periodically, when using the large condenser every 6 hr.; whereas, when using small condensers, their limited capacity necessitates drawing more frequently, for example, every 4 hr. In drawing the condensers the top hole is spiessed first to relieve the gas pressure within and then the scratcher is inserted in the bottom hole and the contained metal and blue powder are drawn with regulation draw car and ladle.
The redistilled spelter in the ladle is cast into plates which are taken to the equalizing furnace for remelting and recasting into the finished plate of uniform high-grade spelter. As the bath of metal in the retort becomes enriched in lead and iron, it must be removed. This is accomplished periodically by omitting the charge for a few periods on one section (nine retorts) daily and the next day by taking down the condensers from this section and scraping out the lead bottoms with a large scraper. Two or 3 hr. are required to clean out bottoms. In case of leaky retorts the
condensers are taken down and the contents of the retorts drawn in the same manner; a new retort is put in and operations continued. The leady bottoms are drawn into a slag pot from which they are cast into plates and taken to a remelting furnace where any excess zinc is separated. The lead tapped from the remelting furnace, carrying about 1 to 2 per cent, zinc, is sold to lead refiners. The blue powder, skiminings, etc., from the redistilling, remelting and equalizing furnaces are returned to the ore furnaces for resmelting. All scrap is returned to the remelting furnace to be recast into sticks for recharging. The crew for operating a redistilling furnace consists of the following men per 12-hr. shift: one first charger who charges the sticks and draws the lead bottoms; one second charger who brings up sticks, stuffs condensers, stacks plates, etc.; one metal drawer who draws metal only; one helper who helps draw bottoms, change retorts, etc.; and one fireman who tends both furnaces in the block and acts as foreman of the labor.
De Saulles Method
The de Saulles process uses a specially constructed furnace equivalent to one side of an ordinary furnace block. The retorts are inclined about 7 in. (17.8 cm.) in their length and extended through the back wall 4 to 5 in. An opening is provided at the top of the protruding butt end through which the retort is charged with molten spelter; at the bottom of

the butt end a small opening is provided through which the leady bottoms are tapped out; both openings are tightly closed with clay except when charging and tapping. The condenser used is of the ordinary shape and size and is clayed into the retort.
The furnaces are fired with natural gas or coal, Belgian style, using a thin flame and natural draft. The furnaces are two, three, and four rows high and contain from 80 to 200 retorts. Each retort-distilling furnace is served by one 25-ton remelting furnace and one 25-ton equalizing-furnace ; the three furnaces are built close together and comprise a redistillation unit. One equalizing furnace may be dispensed with by operating two units together. Fig. 2 shows the method of charging the retorts. The spelter to be redistilled is melted down in the remelting furnace, where a large part of the lead settles out. After settling, the metal is drawn off from the top of the molten bath into a ladle by cutting down a clay retaining dam. The ladle suspended from a trolley is moved along the back wall of the retort furnace and by means of a chain block raised and lowered to the necessary height for emptying its contents into the butt end of the retort, as shown by Fig. 2. The retorts are charged in this manner once in 24 hr.; the two top rows at 7 a.m. and the two bottom rows at 7 p.m. The charge openings are closed with clay packing immediately after pouring in the metal, and distillation of the zinc is resumed with a minimum delay, as the furnace is maintained at a uniform heat throughout the 24 hr. The condensers are drawn every 4 hr. Great caution is exercised in spiessing the stuffing before drawing in order to avoid the spurt of zinc and blue powder caused by the high gas pressure frequently occurring in the condenser. The draw ladle containing the redistilled zinc is moved along an overhead trolley to the equalizing furnace where its contents are emptied. The equalizing furnace serves the purpose of cooling the overheated metal from the condensers and blends the metal from the various draws into car lots of uniform spelter. This furnace is drawn during the day shift only, the metal being drawn off into a ladle by cutting down the retaining dam at the surface of the metal. The refined spelter is transferred on an overhead trolley to the mold racks and cast into finished plates of high-grade spelter. The leady metal is tapped once in 48 hr., half of the retorts being tapped each 24 hr.; about 20 lb. are removed from each retort. Every 10 days the retorts are tapped completely dry and the leady metal obtained is returned to the remelting furnace. The tapping operation takes place about 2 hr. before charging, in order that the lead returned to the remelting furnace may have time to settle out and may not be returned with the next charge metal. Every 2 or 3 weeks the accumulation of lead settling in the bottom of the remelting furnace is tapped out through a bottom tap and cast into molds. This lead containing 1 to 2 per cent, zinc is shipped to lead refiners. All redistilled scrap is returned to the equalizer and leady scrap to the remelter. Blue powder, dross and skimmings are resmelted on the ore furnaces. The labor, per 12-hr. shift, required to operate a double unit of furnaces, consists of one metal drawer and two chargers; on the day shift an extra man is used to help change retorts, etc. One fireman tends two double units. The metal drawer does nothing but draw the condensers and pour the metal into the equalizer; one charger handles the ladle for charging and drawing leady metal; the other charger stuffs the condensers, helps the first charger and draws the equalizer. Both chargers charge the remelting furnace.
Results in Refining by Redistillation
While these two methods of redistilling spelter differ in furnace design and consequently in operation, the grade of impure metal treated, the purity of the product, percentage loss, and percentage of byproducts produced are practically the same; hence the following data pertain to both methods except when the contrary is noted.
Grade of Metal Charged
The spelter ordinarily charged to redistilling operations consists of second- and third-draw metal and contains 1.5 to 3 per cent, lead, 0.03 to 1.0 per cent, iron and 0.03 to 0.07 per cent, cadmium. In the case of the converted smelting furnace method, metal of such composition is charged direct to the redistilling pots, but in the de Saulles method the preliminary settling in the remelting furnace results in metal carrying about 1 per cent, only of lead and 0.03 to 0.05 per cent, of iron being charged into the redistilling pots.
Grade of Refined Zinc
The redistilled spelter contains from 0.03 to 0.12 per cent, lead, 0.008 to 0.012 per cent, iron and 0.03 to 0.07 per cent, cadmium, averaging 0.10 per cent, lead, 0.01 per cent, iron, 0.04 per cent, cadmium; 99.85 per cent, zinc, and is a much better grade of spelter than the ordinary Intermediate grade as specified by the American Society for Testing Materials. In producing such low-lead spelter, it is necessary to maintain at all times in the redistilling pots a large preponderance of zinc, for the amount of lead coming over depends upon its partial pressure and the effect of mechanical entrainment. The more rapidly the zinc is distilled, the more violent the ebullition, and consequently the greater the amount of lead carried over into the condenser by mechanical entrainment. This entrainment effect is frequently observed when a “hot spot” develops in the furnace, or the furnace is being driven for increased production, even though the pots have just been recharged with fresh zinc. Hence the method of controlling the lead content of the redistilled product consists in maintaining at all times a uniform heat throughout the furnace, frequently refilling the retorts with fresh zinc and frequently tapping off the leady metal concentrating in the bottoms. The retorts as inclined in the furnaces hold from 250 to 300 lb. (113 to 136 kg.) of molten metal.
In the de Saulles furnace, about 175 lb. of metal, containing about 1 per cent. Pb, are charged per retort at one time in 24 hr., and the equivalent of 15 lb. per retort of leady zinc containing 3 to 6 per cent. Pb is tapped from the bottoms every 24 hr. In the converted smelting furnace method, about the same amount of metal, containing about 2 per cent. Pb, is charged per retort per 24 hr., but in 6-hr. intervals; about 200 lb. of leady bottoms containing 10 to 15 per cent, lead is drawn every 15 or 20 days, or the equivalent of 15 lb. per retort per day. Because of the frequent replenishment of the retort with spelter, although higher in lead, the metal in the converted smelting furnace retort does not reach a higher lead concentration than that of the de Saulles process retort filled but once per day with lower leaded zinc. The higher-lead bottoms drawn from the converted smelting furnace retort are obtained by omitting charging for a day, and maintaining distillation until a high concentration of lead is effected. Careful control of the lead suffices to keep the iron content in the redistilled spelter ordinarily under 0.01 per cent. Practically no separation of the cadmium takes place; all of it comes over with the zinc.
Yield of Refined Zinc
The average yield of redistilled spelter per retort per day by both methods is about 150 lb. (68 kg.), although as much as 166 lb. (75 kg.) have been produced. The frequent charging of small amounts of cold metal in the converted smelting furnace method avoids overchilling the bath, and interrupts distillation for only a few minutes. Approximately the same conditions are obtained in the de Saulles method through having the larger quantity of charge metal in a molten condition when charged. Drawing off the lead is a much simpler operation and attended with less interruption of distillation with the de Saulles method.
Byproducts
Besides the more or less scrap, skimmings and dross attending remelting and pouring charge and redistilled metal, there is the ever-present formation of blue powder in the condenser; and as this blue-powder product is augmented by frequently opening the condenser, a decided advantage is obtained by using a large condenser, which requires less frequent drawing. The rich uniformly heated zinc vapor from the redistilling pot does not give the condensation troubles that occur with the lean unevenly heated vapors from an ore charge. The proportion of metal going into the byproducts, blue powder, scrap and skimmings, is about 15 per cent, with both processes. The distribution of metal charged to redistillation operations averages approximately:
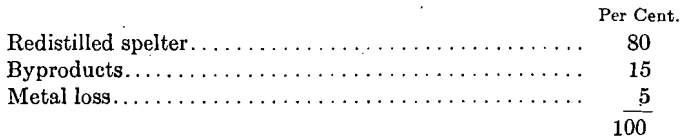
The greatest source of metal loss is with the retorts. The heavy load of metal which the retorts carry at a high temperature sets up strains which only the best made retorts can withstand. Small cracks formed in drying and annealing, or in the retort furnace, rapidly open up, permitting the molten metal or zinc vapor to escape into the combustion chamber of the furnace where it is carried off through the stacks as oxide. On this account, careful and frequent inspection of the furnaces must be made for leaky retorts. Also, the more dense the walls of the retort are made, the smaller the amount of permeation by the molten metal and vapor. It has been found that retorts made in a hydraulic press under the heaviest pressure give the least loss of metal and longest life. The average life of retorts made by the Wettengel hydraulic press has been better than 60 days, as compared with 20 to 30 days of retorts made in other presses. No flame appears on the condenser under ordinary firing, and hence no loss of zinc occurs through escaping uncondensed vapors. Such loss as occurs at the condensers takes place during drawing and charging; a slight loss of zinc occurs during charging by either method.
Redistilling Methods
Jacob Collman and Rudolph Bowmann, propose a continuous process of refining zinc by redistillation, which consists in melting the spelter, passing it in a molten condition and in a thin stream or layer through a suitable conduit placed under the melting pot and subjecting it to a gradually increasing heat in this conduit until distillation temperature of the zinc is reached. The zinc vapor is led off into a condenser where it is condensed into the refined spelter, the molten lead and iron are trapped out of the zinc vapors, and flow off into a separate receiving vessel. The conduit may be filled with pieces of fire-resisting inert material or coal in order to insure a perfect distribution of the zinc to be distilled.
Various other methods of carrying out the redistilling operation have been proposed; for example, extensive experiments have been made with furnaces heated electrically for the purpose of closer temperature control in order to affect separation of the cadmium as well as lead and iron. Others have tried systems of countercurrent heating and conserving the latent heat of condensation of the zinc to effect separation of the cadmium, etc., but such efforts have not so far developed a commercial process.
Methods of Refining by Remelting
Remelting or tank furnaces are used by several smelters for equalizing the composition or for refining spelter by remelting and settling out the lead and iron. Lots of spelter containing under 0.80 per cent, lead, or that will grade as Brass Special, are remelted in a remelting furnace, holding about 30 tons of molten metal. This is a simple remelting operation which serves to equalize the composition of the small lots produced from different parts of the retort furnace and yields uniform spelter in carload lots. Lots that contain over 0.80 per cent. lead, or which grade as Prime Western spelter, are remelted in another furnace. The plates are slowly melted in the furnace and after standing 18 to 24 hr. to permit the lead and iron to settle, the top metal is drawn off and the tank replenished with more plates. In this way, a certain amount of refining can be accomplished; for example, the lead content of the top metal may be lowered to 0.90 to 1.20 per cent, and the iron to 0.030 to 0.040 per cent. As these remelting furnaces fill with lead and iron, the bottoms are tapped and the heavy metal run out, or the top zinc is drawn off and the heavy metal dipped out. The separation of the lead in these furnaces depends upon the fact that its specific gravity (11.4) is higher than that of molten zinc (6.86) and upon its decrease in solubility in zinc at the melting temperature of the latter. Iron separates in the form of an alloy (hard zinc containing 5 to 6 per cent. Fe) and collects on top of the lead. This hard zinc is removed from time to time with a perforated shovel and returned to the retort furnaces with the dross and skimmings.
Remelting furnaces are built with a deep basin for the purpose of maintaining the lead accumulation, between infrequent clean-out periods, well below the top zinc, in order that it may not be stirred up by the surface agitation during charging and drawing. The basin is frequently divided by two or three partition walls with slots near the metal level to permit only top metal to flow from one compartment to the other. A sheet-iron shell to prevent metal leaks incloses the bottom and extends around the sides to the metal level. Furnaces are fired by gas or coal, using a reducing flame, the flame being held close to the surface of the metal by means of a low arch. Plates of spelter to be remelted and refined are charged at the fire end, either in a separate compartment or on a sloping hearth, and the refined metal is drawn from the last compartment at the other end. Drawing is accomplished either by dipping with a ladle or by cutting down a dam and allowing the top metal to flow into the ladle; the metal is then cast into finished plates of uniform spelter. Control of the quality of the refined spelter consists in slowly melting the charge plates, keeping the temperature of the metal at the drawing end slightly above the freezing point, not permitting the lead and iron accumulations in the bottom to get within 10 to 12 in. of the surface of the metal and maintaining the surface of the metal at practically the same level when operating continuously; that is, in not drawing out more metal than is put in and slowly melted. The leady metal from the bottoms, carrying about 97.5 per cent, lead, 2.5 per cent, zinc, and 0.01 per cent, iron, is shipped to lead refiners. The distribution of the zinc contents in the production of 1 per cent, lead spelter is approximately:

Other methods have been proposed for refining zinc in the molten condition, such as introducing other substances which would combine with the impurities and bring them to the top or carry them to the bottom. Delfo Coder proposes the introduction of copper or copper aluminum alloys into molten impure zinc, and the distilling off of the zinc which the copper or alloys take up. Richards removed the iron from galvanizers’ slab dross, on a commercial scale, by introducing potassium cyanide and sulphur into a bath of impure zinc. The iron settled to the bottom as sulphide. He also accomplished the same result by using leather scrap and potatoes as substitute for the potassium cyanide.
