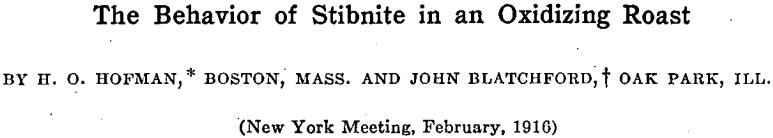The leading antimony mineral is stibnite. In smelting stibnite ore two processes are available, precipitation and roasting-reduction. The former is suited, only for high-grade ores. As low-grade ores are more common than high-grade, roasting-reduction is of greater importance than precipitation. In the roasting process the aim may be to leave the oxidized antimony in the ore, or it may be to volatilize as much of the antimony as possible, collect the volatilized oxide as a rich intermediary product and smelt it for antimony, leaving the gangue poor enough to be considered a waste product.
Whichever way the roast is conducted certain difficulties inherent in stibnite are encountered. These are:
- The low melting point of stibnite which; according to Pelabon, is 550°C., according to Wagemann 540°C., and according to Borgstrom 546°C.
- The ignition temperature: According to Friedrich, stibnite, if heated in air, begins to oxidize at 290°C. if the size of a grain is 0.1 mm. in diameter; at 343°, if 0.1 to 0.2 mm.; and 430° if 0.2 mm.
- The fusibility of a mixture of Sb2S3 and Sb2O3 which in the form of kermesite (Sb2S3)2.Sb2O3 melts at 517°C.
- The volatility of Sb2S3 and Sb2O3, for which no numerical data appear to exist, although practical experience has shown that they are volatile at low temperatures.
As regards the oxidation of metallic antimony, we have the experimental evidence of C. F. Plattner that when fused and brought to a red heat, it burns with a bluish-white flame to Sb2O3 which passes off as a whitish fume, and that Sb2O3 can be further oxidized to Sb2O4 or Sb2O5 If Sb2O4 is considered to be antimonious antimonate, the compound falls in line with metallic antimonates which are formed if a metallic antimonide or sulphantimonide such as pyragyrite, tetrahedrite, or jamesonite, is subjected to an oxidizing roast—or if Sb2O3 in contact with a finely divided metallic oxide is heated in a current of air.
The chemical change which takes place in roasting stibnite is usually expressed by the equation
Sb2S3 + 9O = Sb2O3 + 3SO2
but the Sb2O3 is further oxidized to. Sb2O4. In carrying on a roast, the temperature of the ore is held at first at about 350°C., and the charge is rabbled more or less continuously to prevent or correct caking; later, when part of the Sb2S3 has been converted into Sb2O3 and Sb2O4, the temperature may be raised with advantage. Roasting changes the black sulphide into a yellowish-white oxide.
The aim of the present investigation was to study the changes stibnite undergoes when roasted at different temperatures; to determine the composition of the roasted product; and to ascertain the losses which occur.
The material used was crude stibnite of unknown origin from the metallurgical collection of the Massachusetts Institute of Technology. It was a compact grayish crystalline mass showing fine prismatic needles. The fresh fracture appeared silver-white and had a metallic luster. The general appearance was not unlike that of graphite. After grinding, it had a jet-black color. A chemical analysis gave 71.66 per cent, antimony and 0.96 per cent insoluble. Calculating the antimony as the sulphide gives 100.3 per cent. Sb2S3; the excess over 100 is probably due to the presence of a small amount of metallic antimony.
The roasting furnace used was of the electric resistance type. It consisted of a porcelain tube, 10 in. long and 2¾ in. in diameter, wound with “nichrome” wire; this was surrounded by mineral wool, and the whole inclosed in a pipe of galvanized iron, 7½ in. in diameter. Temperatures were measured with a thermo-electric pyrometer.
The ore was ground to pass a 20-mesh sieve; a 5-gram sample was used, as this covered the bottom of the porcelain boat and filled it to about one-third of its depth.
During a roast the ore was frequently rabbled with a wire bent to the form of a hook. A piece of moistened litmus paper held above the surface of the ore served as a test for sulphur dioxide. Any odor, or change in the appearance of the ore was noted. The temperature of the furnace was raised gradually to the point at which it was desired to finish a roast, and held there until no more changes in appearance and weight of the charge occurred.
Seven experiments were carried out, covering a range of finishing temperatures from 337.5 to 545.4°C. Experiment No. 3, in Table I, shows the manner in which the records were taken.

A summary of the observations made during the six roasts is given in Table II.

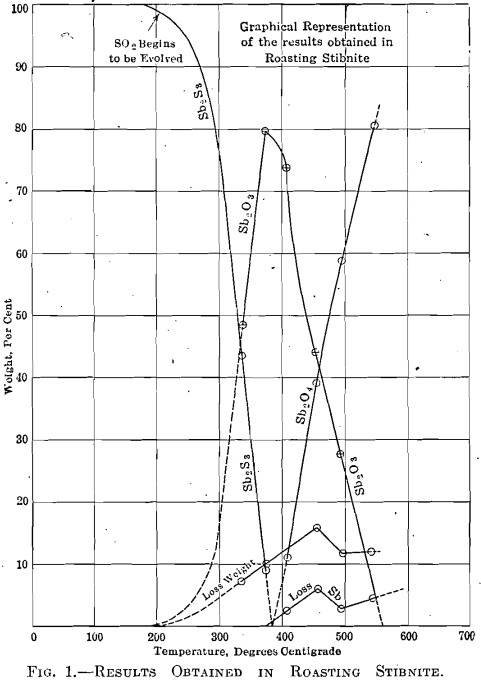
The first observation made in each roast was the evolution of sulphur dioxide; this was followed by a change in the color of the litmus paper at a slightly higher temperature. At 280°C. the ore began to lose its luster and became dull. Then followed a strong odor of sulphur dioxide which directly preceded a sudden visible formation of Sb2O3 at 336°C. The oxide forms spontaneously at this temperature in the form of little yellow grains which become white upon cooling. As the temperature is raised above 336°C., the grains seem to break up into a grayish powder. If the temperature is raised too quickly before all the sulphide has been changed into whitish grains of oxide, the charge becomes sticky; this is not the case if the temperature is raised gradually. In roasts Nos, 6 and 7 a small amount of Sb2O3 was deposited on the rim of the heating tube, but the temperature and time at which the deposit was formed were not noted. Tables III, IV, and V contain data on the roasting experiments and the results, obtained.
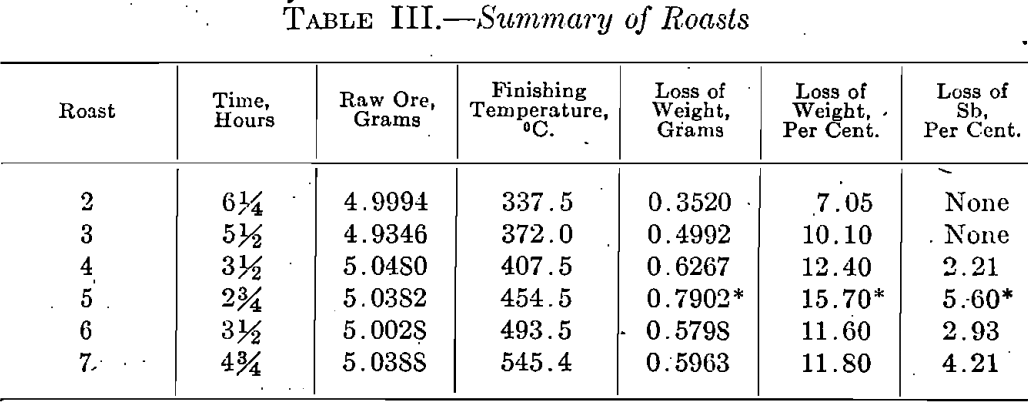
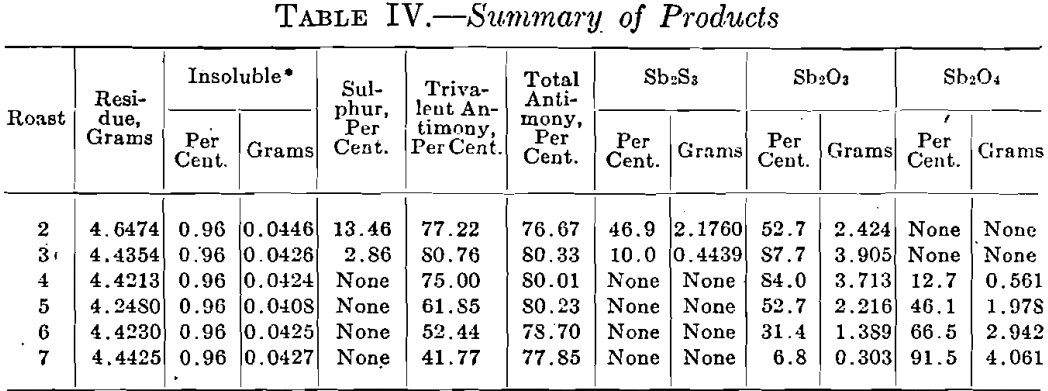
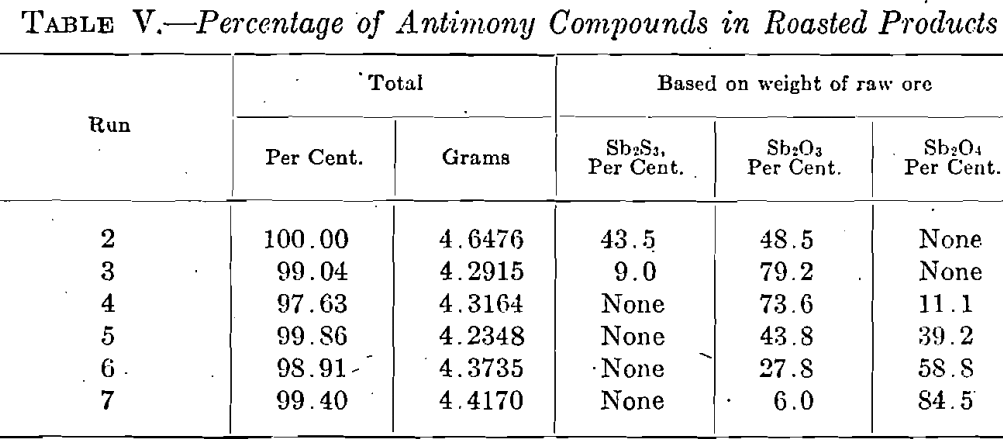
A graphical representation of the results, given in Fig. 1, shows clearly the changes which stibnite undergoes when it is roasted. No change takes place until the gradual rise in temperature has reached 200°C. when SO2 begins to be given off. The quick fall of the Sb2S3 curve shows that beginning with 200°C. a rapid oxidation takes place which is terminated at 400°C. It is fair to assume that the formation of Sb2O3 commences at the temperature when SO2 is first detected. The Sb2O3. curve is therefore started at about 200 °C; it has a rapid rise which corresponds to the quick fall of the Sb2S3 curve. Its highest point coincides on the temperature scale with the point at which the oxidation of Sb2S3 is complete; the Sb2O4 curve starts at the same temperature. As the temperature rises, the Sb2O3 curve falls and is accompanied by a corresponding rise in the curve for Sb2O4. From this it is seen that in roasting Sb2S3 there is formed at first only Sb2O3, and that this is converted into Sb2O4 only when all the Sb2S3 has been oxidized. Thus the amount of Sb2O4 formed is dependent upon the temperature and the time of the roast. It is assumed that there is no loss of weight below the temperature at which SO2 begins to be evolved, i.e., about 200°C. From this point on, the loss in weight increases with the temperature and the formation of Sb2O3; it reaches a maximum at about 450°C. then falls and afterward becomes nearly constant. The loss in antimony appears to be a function of the temperature; the curve shows that it increases with the finishing temperature with the exception of the jog at 450°C. which, as stated with Table III, is an unexplained experimental accident.
The following conclusions may be drawn from the investigation:
- The ignition point of stibnite is approximately 200°C. as evidenced by the odor of SO2 evolved and its action on litmus paper.
- There is a sharp visible formation of Sb2O3 at 336°C. At this point white fumes are evolved.
- The formation of Sb2O3 takes place slowly, probably according to the reaction:
Sb2S3 + 9O = Sb2O3 + 3SO2 - It is possible to eliminate all the sulphur slightly below 400°C. without a large loss of antimony.
- Stibnite is oxidized at first to antimony trioxide which begins to change to antimony tetroxide only when all the sulphide has been decomposed.
- The amount of tetroxide increases as the trioxide decreases, the. action being more rapid as the finishing temperature is raised.
- The loss of antimony during the roast increases with the temperature.
A discussion of the chemical work is given in the paper by W. T. Hall and J. Blatchford, read at this meeting.