The so-called “combination method” is generally used in assaying bar copper for silver. It has been modified from time to time. Briefly outlined as now practiced, it is as follows:
One A. T. of the borings is dissolved in dilute nitric acid. When solution is complete the liquid is boiled and then filtered to remove gold. The filtrate is treated with sufficient salt solution to precipitate all the silver, but avoiding any unnecessary excess. The liquid is allowed to stand overnight and next morning the silver chloride is collected on a fresh filter, which, together with the paper containing the gold and insoluble matter, is scorified and cupelled. Formerly many assayers added sulphuric acid to the nitric acid solution of the copper and silver and then acetate of lead, thus producing a heavy precipitate of sulphate of lead which was supposed to entangle the silver chloride and prevent it from passing through the filter. As a matter of fact the use of sulphuric acid and lead salts is entirely unnecessary. Very few assayers now make use of them. If it is not possible to let the silver chloride settle over night, accurate results may be obtained by stirring the liquid vigorously with some form of mechanical stirrer for half an hour. The silver chloride may then be easily filtered without fear of any of it escaping through the filter. The same result may be obtained by blowing air through the liquid. It is quite possible to make an accurate assay by this method in three hours. In some cases, noticeably those in which the copper is very free from impurities and the gold contents small, the correct gold assay may be obtained by parting the bead obtained by this process. In many cases, however, the gold assay obtained by the above process is too low, even though the gold be removed from the liquid by filtration before adding the salt solution. It would appear that sometimes the gold is present in the copper in some combination which is soluble in nitric acid or strong nitrate of copper solution. However, it may be, and frequently is, necessary to resort to the “ all-fire method ” to obtain correct results. This method consists in weighing out a number of 1/10 A. T. portions—usually ten—and scorifying them with lead until most of the copper is removed—then cupelling the lead-buttons either separately or uniting them five and five, rescorifying, and then cupelling. This method is expensive and laborious, involving many scorifications and the use of much test-lead. The first scorification must be conducted at a high temperature, and the operations consume a great deal of time and muffle-room.
But the gold obtained is usually appreciably more than can be extracted by the combination-method—though it does not seem to bear any fixed ratio to it even in the same class of copper. In general, in 96-98 per cent, copper, containing from 1 to 5 oz. of gold per ton, the “ all-fire” results will be from 0.1 to 0.3 oz. higher than can be obtained by the combination-method. Any method that would give correct gold and silver results on all classes of bar-copper at one operation, and that would avoid the tedious and expensive operations of the all-fire process, would find ready acceptance among assayers. The method described seemed to offer some possibilities in this direction. To test the practical accuracy of this method, comparative assays were made in my laboratory on three samples of bar-copper, using the “ combination-method” for silver, and the “ all-fire ” process for gold, alongside of the method. Some preliminary experiments were made by the new method to obtain a knowledge of any peculiarities that might be developed. The process of assay was as follows:
One A. T. of borings dissolved in dilute nitric acid (90 c.c. strong to 100 c.c. of water). The solution was then evaporated to expel free nitric acid, a little sulphuric acid (20 c.c.) was added, and the evaporation continued. Finally the copper salts were dissolved in hot water, the solution diluted to 800 c.c., and allowed to cool. Then sulphuretted hydrogen was passed rapidly into the liquid for two minutes. A heavy black precipitate of sulphides was produced, which settled rapidly, leaving a clear blue solution. The liquid containing the precipitate was stirred rapidly and then allowed to settle for about half an hour, and finally the sulphides were filtered off. The filtrates in most cases gave no visible reaction for silver; sometimes, however, silver was not completely precipitated by the sulphuretted hydrogen. No reason was developed why this should be the case, as those solutions which contained silver had apparently been treated exactly like those which did not. The assays noted below did not show any reaction for silver when the filtrate was tested with salt-solution. The copper sulphide containing the silver and gold was dried, the papers burned in scorifiers, and the residue scorified with 50 grammes of test-lead.
The results were as follows :
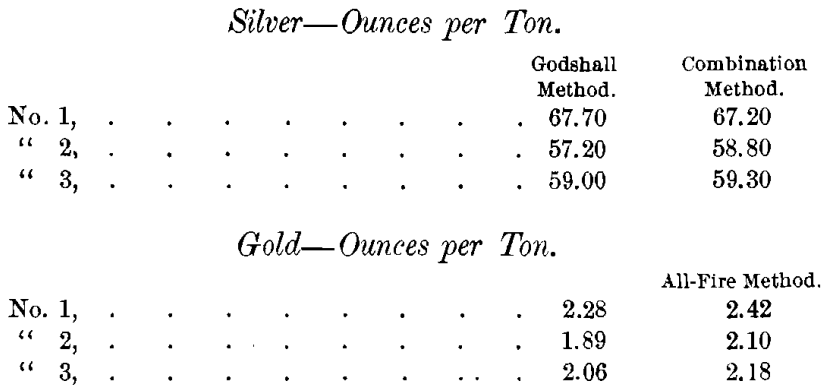
From the foregoing results one would infer that the new method can give good results on silver. But the gold-assays are too low, being about the figures which would be obtained by parting the silver-beads obtained by the “ combination- method.” The new method does not seem to possess any advantage over the “ combination-method ” in point of speed. When the silver chloride is stirred well or “ blown down ” with air, it can be filtered off as quickly as the sulphide precipitate. On the other hand, the new method requires the use of sulphuretted hydrogen, which is a noxious and troublesome reagent to handle on a large number of samples at the same time.
The metallurgical public has been placed under great obligations by reviving the subject of the determination of gold and silver in copper-bearing materials. Several years ago Dr. Ledoux brought this subject before the Institute in a valuable paper, but I have always felt that the subject was not as fully discussed at that time as might have been done with profit. It was clearly shown by the results then reported that the “ combined ” method yielded lower gold-results than the fire-assay. This was supposed to be due to a solution of some of the gold, occasioned by the impurities in the nitric acid used; and it seems to have accepted that view. Since then, I have determined by experiment that when the acid is chemically pure, a partial solution of the gold takes place, probably by reason of the formation of nitrous acid during the process of solution, and that the reaction, though manifested in smaller degree, is closely allied to that which occurs when platinum, in the form of an alloy, is dissolved in nitric acid. This being admitted, it is clear that the “ combined ” method cannot be relied upon for gold. We were therefore greatly in need of a method such as the one proposed. I have experimented with it, and find that, under certain conditions, excellent results are obtained. The conditions for satisfactory working are given in his paper; but I do not think the danger-points are clearly marked. I found that, when the solution was cold, and practically free from nitric acid, the precious metals were perfectly thrown down with a very small amount of H2S. In such cases, the amount of copper to be removed by scorification and cupellation was small, and gave no trouble; but when the copper-solution contained much free nitric acid, or was hot, large amounts of H2S were required to complete the precipitation; this had to be followed by a rapid filtration; and, even then, the solution frequently contained traces of silver. The removal of the nitric acid by evaporation with sulphuric acid is a matter of considerable difficulty, as I believe all who have tried it will admit. This and the careful attention required in making the H2S precipitations are the chief objections to the method. I have modified it with good results as follows :
I dissolve in HNO3; dilute and make faintly alkaline with ammonia; then make acid with acetic acid. I then add a solution of hypo-sulphite, sufficient to precipitate about 100 mgs. of copper. After thoroughly mixing the solutions with a stirring rod, the beaker is boiled for five minutes, or until the Cu2S collects. This precipitate is cupelled in the usual way. With this modification of the proposed method, I believe there remains only one objection, viz., the necessary scorification of a product high in copper. I have been working with some success to overcome this difficulty; and I hope to publish the result of my efforts hereafter.
