Table of Contents
- PREPARATION OF SODIUM & CALCIUM SULPHIDES
- FORMATION OF HYPOSULPHITE SALTS
- Producing Hyposulphites by Oxidation of Sulphide Solutions
- Precipitation of Calcium by Sodium Sulphide Solutions
- Regeneration of Sodium Hyposulphite From Tetrathionate
- PRECIPITATING COEFFICIENTS
- Precipitating-Coefficients for c. p. Reagents
- Precipitating-Coefficients far Commercial Reagents
- Precipitation of Lead and Calcium by Solvay Soda
- Weights of Precipitates and their Percentages in Metals
- Financial Results
- Application of the Tables to Special Cases
- EXAMPLE
- The Loss of Hyposulphite in Lixiviation
Metallurgical processes cannot be conducted successfully without the aid of analytical chemistry. The great perfection of lead-smelting in the West, for instance, has only been accomplished by the analysis of ores, fluxes, slags and all products of the furnace. Ores are mixed and fluxed to obtain a slag of desired composition. Account is even taken of the composition of the ashes obtained from the coke. The “ muscular ” smelter has left the field forever! This state of affairs has been induced by sharp competition, i.e., by a complete separation of the industries of mining and reducing ores, not by the liberality and wisdom of directors and stockholders to provide laboratories and engage chemists, or by their love for scientific investigation.
It would be considered absurd, at present, to run a lead-smelter, a blast-furnace for pig-iron, or a Bessemer plant without the assistance of a well-equipped laboratory and a chemist; but it is considered quite sufficient to provide amalgamation- or lixiviation- works merely with a crude assay-office, and an assayer who is paid less than a laborer in the mill.
In my opinion, this will change in the near future. By the exclusion of Mexican lead-ores, and their growing scarcity in the West, smelters have been forced to raise their charges on so-called dry silver-ores, and cannot handle the latter now in such unlimited quantities as formerly. The inevitable result must be that the surplus of these dry silver-ores, especially those of low grade, will be reduced by processes cheaper than smelting; and here the introduction of lixiviation has an excellent field, provided the muscular lixiviator has an able chemist for an assistant.
It is to be regretted that so little analytical work has been done in lixiviation. We know almost nothing of the composition of roasted ores before and after lixiviation; of the constitution of the first wash water and of lixiviation-solutions after prolonged use; of the chemistry of sodium and calcium sulphides; of the composition of sulphide and carbonate precipitates. Without such work material progress is not possible.
During the last seven years a great number of papers on lixiviation have been published, containing a good deal of valuable information, but also many errors due to hasty and incomplete investigation without thorough analytical work. Writers of metallurgical treatises, myself included, have repeated these errors like parrots—what else could they do! I will point out a few. We find it almost everywhere stated that in boiling caustic milk of lime with sulphur the lower calcium polysulphides cannot be produced, because they are insoluble. This is not correct. CaS cannot be obtained in the wet way or in solution. If it is made by heating CaCO3 in fumes of CS2 and CO2, and brought in contact with water, CaH2O2 and CaH2S2 are formed) the latter being readily dissolved. According to the best authorities, CaS2 and CaS3 do not seem to exist at all, at least not in aqueous solution. If caustic lime is boiled with sulphur, the tendency prevails to form CaS4 even if an excess of lime is present, not because CaS2 and CaS3 are insoluble compounds, but because calcium has stronger affinities to unite with four equivalents of sulphur.
A more serious error occurs in Daggett’s paper, Trans., xvi., 428, in regard to the abnormally high precipitating-coefficients of sodium sulphide prepared according to Russell’s directions. Believing in the correctness of Russell’s experiments, I built upon them an elaborate theory in my book on the Lixiviation of Silver-Ores with Hyposulphite Solutions. Aaron was the first who proved that these abnormal precipitating-coefficients exist only in partial precipitation, which Russell had entirely overlooked; hence, they have no existence in practical millwork. That Aaron’s results are correct, I know from personal observation. In the following I have to record another important error. In Daggett’s paper, already quoted, we find on page 430 that calcium monosulphide is precipitated by Russell’s sodium sulphide if the hyposulphite solution contains calcium salts. The yellow precipitate of calcium sulphide is said to appear after nearly all the silver, copper and lead have fallen out. I have no doubt the precipitate occurs, and looks yellow; but it is not CaS, or CaS2, or any other calcium sulphide. Like all other faithful writers of books, I have also endorsed this statement in my treatise on lixiviation.
The metals playing an important part in precipitation from hyposulphite solutions, resulting from the lixiviation of silver-ores, are silver, copper, lead, and calcium. The quantity of gold that may be present is too small to enter into calculation. Silver and copper are always precipitated as sulphides by sodium or calcium sulphide; lead and calcium may be precipitated as carbonates by Solvay soda; or lead alone as hydroxide by caustic lime. Sodium sulphide can be used either as a mixture of Na2S and NaS2, which we will designate in future as “ Russell’s Sulphide,” or NaS2, or a higher poly-sulphide. Calcium sulphide is always used as CaS5. For precipitating lead, caustic milk of lime is prepared with hyposulphite stock- solution. Solvay soda is also dissolved in stock-solution.
Calcium may also be precipitated by a sodium sulphide solution, as will be fully discussed later on. Caustic lime and Solvay soda are always used before calcium or sodium sulphide.
In the absence of calcium, the lead carbonate precipitated by Solvay soda can be obtained practically free from silver, that is to say after washing it with fresh hyposulphite solution. From Ontario ore, for instance, Russell obtained in this way lead carbonate with only two ounces of silver per ton. If calcium is present, the lead-calcium carbonate contains more or less silver, and may be very rich. According to Wilson and Russell, the silver does not increase in proportion to the calcium precipitated. Russell also claims that washing the precipitate with pure or more concentrated hyposulphite solution will reduce its value in silver considerably.
This is not clearly established. At the Marsac mill, one lot of carbonates was recently produced containing 8.1 per cent, lead, and 392 ounces silver per ton; and another lot containing 19 per cent, lead, and 1269 ounces silver per ton. I do not know the exact circumstances under which these carbonates were obtained. Evidently, this subject, like so many others in lixiviation, needs a thorough analytical investigation.
Lead hydroxide precipitated by caustic lime is generally rich in silver. According to Rueger, the lead precipitate obtained at the Mount Corry mill, Nevada, contained 60 per cent lead and 420 ounces and more of silver per ton.
The separate precipitation of lead and calcium may be not only economical directly, but also indirectly by the production of silver sulphides of higher grade.
Since the introduction, by Kiss, of CaS6 as precipitant, the majority of metallurgists have tenaciously adhered to this practice, evidently without examining the subject critically. The question has become more complicated by the recent introduction of methods for separate precipitation of lead, and of the Russell process. It is hardly necessary to add that the problem also includes the question whether a sodium or calcium hyposulphite solution deserves preference in lixiviation.
Although lixiviation is now always commenced with sodium hyposulphite, this salt is gradually replaced by calcium hyposulphite if CaS5 is used as precipitant.
In the following I propose to present in a systematic manner the most important facts that have a practical bearing upon the subject of precipitation. The reader, however, should not expect a complete and exhaustive treatise, for which analytical data are entirely lacking.
PREPARATION OF SODIUM & CALCIUM SULPHIDES
Both reagents are prepared by boiling either a concentrated lye of caustic soda, or caustic milk of lime with sulphur. In making calcium sulphide, sufficient sulphur must be added to the caustic lime to obtain CaS4, because lower calcium sulphides are not formed. In fact, the solution contains CaS4 even if an excess of lime is present, while an excess of sulphur produces CaS5. From the slight solubility of calcium hydrate in water it follows that this process must require considerable time, and that at the end of the operation, a solution of only moderate concentration must result. From sodium hydrate, on the contrary, a lye of almost any concentration can be obtained, as well as sodium monosulphide, and all of the polysulphides. Hence, the process must be completed very rapidly, and, if the lower polysulphides are desired, with a minimum consumption of sulphur.
The mode of preparing calcium sulphide is well known. I will only call attention to the facts that it is troublesome, wasteful in chemicals, amount of steam and time consumed, and that an excess of sulphur should be used to obtain CaS5.
Russell discovered a practical and easy method of preparing sodium sulphide. According to his directions, the contents of a drum of caustic soda are broken into lumps and dissolved with the aid of steam in a minimum of water, thus forming a very concentrated lye. The operation is conducted in a cast-iron tank 3 feet diameter and 7 feet high. When the lye has reached a temperature of not less than 100° C. sulphur is gradually added, two-thirds of the weight of the caustic soda. A most violent reaction takes place, the mass foaming considerably. This, however, is caused by disengagement of steam and not of any other gas. After all the sulphur has disappeared, the product is allowed to cool, dissolved by adding hyposulphite stock-solution, and discharged into a storage-tank, where the solution can be diluted still further. In two hours, 700 pounds of caustic soda, the contents of one drum, can be easily converted into sodium sulphide. The solution contains a mixture of Na2S and Na2S2.
The quantity of sulphur used for making Russell’s sulphide is somewhat empirical. The two-thirds rule may be very convenient for the laborer; but metallurgy has not been invented for his convenience. As will be seen at once, the rule does not at all consider different grades of caustic soda, which may contain from 85 to 95 per cent. NaHO, thus producing .sodium sulphide differing materially in composition. If we use sulphur with 95 per cent. S, and caustic soda with 95 per cent. NaHO, the resulting sulphide will be Na2S + Na2S2. If, however, the caustic soda contains only 85 per cent. NaHO, the sulphide will have very nearly the composition Na2S + 4 Na2S2.
This subject will he further discussed in the following paragraphs :
FORMATION OF HYPOSULPHITE SALTS
The reactions taking place in boiling caustic soda with sulphur are expressed by the equation :
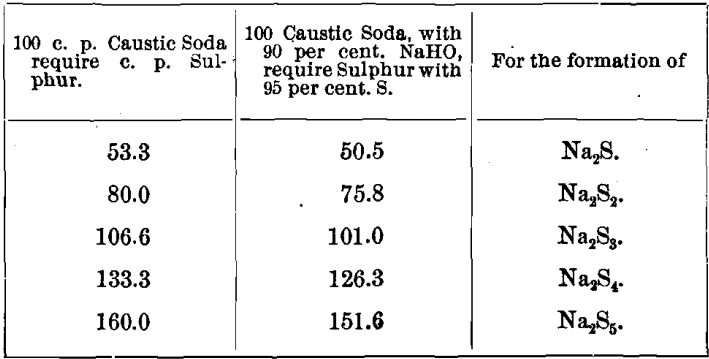
(1.) 6 NaHO + (2 + 2x)S = 2 Na2Sx + Na2S2O3 + 3 H2O.
That is to say, for two equivalents of a sodium sulphide, one equivalent of the hyposulphite salt is formed.
For the formation of different sodium polysulphides, the quantities of sulphur consumed would be as follows:
In manufacturing sodium sulphide there are no very important side-issues to the reactions expressed by equation No. 1; and, taking into consideration the impurities of commercial caustic soda and sulphur, calculations for practical purposes can be based upon them. There are, however, certain limitations to the equation. It is not true, for instance, when x = 1. In using an amount of sulphur only sufficient for the formation of Na2S, caustic soda remains free, and polysulphides are formed besides Na2S. It is even difficult to obtain a reagent absolutely free from caustic soda by using enough sulphur to form Na2S2. Such a solution is also liable to contain some Na2S and polysulphides higher than Na2S2, a tendency prevailing to form the higher polysulphides. If we replace in equation No. 1 sodium by calcium, the reactions become still more complicated. In the first place, the equation is only true for CaS4 and CaS5. It is very probable, however, that some sulphydrates are also formed, and this seems the case even in preparing sodium sulphide. I will here draw attention to the fact that H2S frequently escapes at the end of precipitation, even if the solution has originally an alkaline reaction. This is the case in using both calcium and sodium sulphide as precipitants. The formation of H2S can be easily explained if we assume the existence of sulphydrates. The reaction would be as follows: Ag2S2O3 + CaH2S2 = Ag2S + CaS2O3 + H2S. Naturally, the H2S only escapes when precipitation of the metals is nearly completed.
This also explains an observation made by Russell at the Marsac mill.
He says: “ The solution running from the ore-vats to the precipitating-tanks is perfectly neutral, but after precipitation with sodium sulphide it is so acid that blue litmus-paper is instantly reddened by it. At the end of precipitation the odor of H2S is very perceptible; it even appears during precipitation if stirring is not well done.”
This, it seems to me, shows the presence of sulphydrates. The H2S formed in the beginning of precipitation is absorbed by the solution, throwing down metals and setting acid free. To return to the calcium sulphide:
The reaction is also disturbed by the formation of oxy-sulphurets —which are not easily soluble—especially if sulphur is not used in excess to form CaS5, and by decomposition of calcium hyposulphite at boiling-point. While a sodium hyposulphite solution can be heated to boiling without detriment, that of the calcium salt decomposes above 60° C. as follows :
CaS2O3 = CaSO3 + S.
This decomposition is, however, resisted in the presence of CaS4 and CaS5, and counteracted by the opposite reaction:
CaSO3 + S = CaS2O3,
which takes place between 30° and 40° C., that is to say, after cooling and settling of the solution. To what extent these opposing reactions balance each other is not known, and depends, most likely, upon various circumstances.
For these reasons it is not possible to calculate beforehand, even approximately, from the chemicals consumed, the effect of a calcium sulphide solution in precipitation, or to compare it, merely by calculation, with the same effect of a sodium sulphide solution. Only actual mill-statistics make a comparison between the two reagents possible. It can readily be seen, however, that the preparation of CaS5 must involve a waste of chemicals compared with that of Na2S2.
According to equation No. 1, 100 parts of caustic soda, consumed in the manufacture of sodium sulphide, will produce 103.3 parts of Na2S2O3 + 5 aq.
Or 100 parts commercial caustic soda, containing 90 per cent. NaHO, would produce 93 parts Na2S2O3 + 5 aq. Both sodium and calcium sulphide solutions, if exposed to the air, oxidize with formation of hyposulphite salts, but the latter more rapidly than the former.
In case Na2S is exposed to the atmosphere, sodium hydrate is formed besides hyposulphite:
(2.) 2 Na2S + H2O + 4O =Na2S2O3 + 2NaHO.
The caustic soda is then converted into carbonate by absorbing carbonic acid from the air. Na2S2 is completely converted into hyposulphite, while the higher polysulphides are oxidized with precipitation of free sulphur.
O. Hoffman, who used CaS5 as precipitant at the Silver King mill, Arizona, stated to me that the original stock-solution was used over a year and a half, and that it increased in strength and volume, making it necessary to run a part of it to waste.
In this case a large amount of copper and lead was precipitated with the silver. This is good evidence to show how rapidly CaS5 oxidizes. What has been said about the more rapid decomposition of calcium sulphide in contact with air, compared with sodium sulphide, holds good regarding the respective hyposulphite salts.
Russell exposed solutions of different concentration in soup plates to the atmosphere for seven days, at a temperature of from 20° to 22° C., and brought them to their original volume at the expiration of that time. The relation of the depth of the solution to the diameter of the plates was about 1 to 8. The deterioration which had taken place was for sodium hyposulphite, 1.4 per cent., and for the calcium salt, 16.1 per cent.
Formerly lixiviation-solutions were never heated; the new practice finds it profitable to operate with solutions of a temperature as high as 50° C. Would not the loss in calcium hyposulphite be much increased in such a case, when complete decomposition takes place above 60° C? The stubborn advocates of the old practice in lixiviation say: We never needed to buy sodium hyposulphite; our stock-solution increased in strength, and we had to run it finally to waste! But at what expense, gentlemen, in caustic lime and sulphur, and inferior extraction in silver, did you reach this result ?
Producing Hyposulphites by Oxidation of Sulphide Solutions
The question is of importance, whether it is cheaper to buy and add sodium hyposulphite to a deteriorated stock-solution, or to introduce hyposulphite by allowing the sulphide solution to oxidize, whereby it degenerates in precipitating power, but gains in hyposulphite. This depends upon local prices of chemicals. In most cases it will be cheaper to produce the hyposulphite by oxidization of the sulphide. A calculation under assumed conditions will be of interest. For Na2S2 we find :
The formation of 100 pounds Na2S2O3 + 5aq requires:
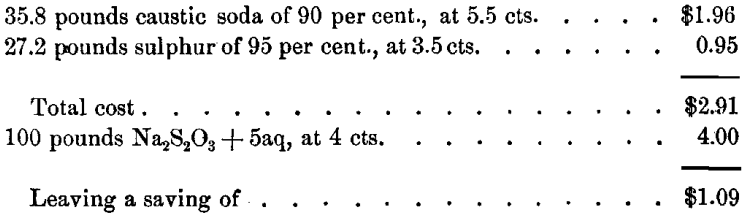
if this salt is obtained by the oxidation of Na2S2 in the mill.
The cost of obtaining calcium hyposulphite from the sulphide solution by oxidation cannot be accurately or even approximately calculated, for reasons already stated. From the fact that CaS5 must be oxidized in place of Na2S2, and that in the preparation of calcium sulphide relatively more chemicals are consumed, it follows that the cost cannot, in most cases, be materially less, although caustic lime is very much cheaper than caustic soda; and it may be even greater.
To use a calcium sulphide solution so highly oxidized that the stock-solution increases in strength and volume, making it necessary to run it to waste, cannot be good economy, because it necessitates an excessive consumption of the precipitant.
Should it be desirable to use an oxidized sodium sulphide solution, containing a large amount of hyposulphite, oxidation is most conveniently and quickly effected by forcing air through a coil of gas-pipe, provided with a great number of small holes, and placed at the bottom of the storage-tank.
Precipitation of Calcium by Sodium Sulphide Solutions
This subject has never been considered or presented before. I have observed in former paragraphs:
- That it is difficult to prepare the lower sodium polysulphides absolutely free from caustic soda.
- That if a sodium sulphide solution containing Na2S is exposed to the atmosphere, Na2S2O3 + 2NaHO are formed.
- That free caustic soda absorbs carbonic acid from the atmosphere and becomes Na2CO3.
In these reactions lies the key to the appearance of the mysterious yellow precipitate which Russell took for CaS. As can readily be seen, if Russell’s sulphide is for any length of time exposed to the atmosphere, it must contain more or less Na2CO3, The Na2CO3 originally contained in commercial caustic soda—which may vary between 3 and 5 per cent.—also comes into play. Assuming, for instance, that 6 pounds caustic soda were consumed (manufactured into sodium sulphide) per ton of ore, containing 4 per cent. Na2CO3, there would come into action 0.24 pound Na2CO3, precipitating 0.09 pound Ca = 0.22 pound CaCO3. Assuming that Russell’s sulphide had been made of a high-grade caustic soda (with 95 per cent. NaHO), having the composition Na2S+Na2S2, and that by long exposure to the air, one-half of the Na2S became oxidized, how much Na2CO3 would a quantity of Russell’s sulphide contain produced from 6 pounds caustic soda? Exactly 1.28 pounds, precipitating 0.48 pound Ca=1.20 pounds CaCO3. Adding the sodium carbonate originally present in the caustic soda, the total amount would be 1.52 pounds, precipitating 0.57 pound Ca = 1.42 pounds CaCO3.
A sodium sulphide solution containing Na2S, if allowed to oxidize, is unprofitable for another reason. Equation No. 2 shows that only one-half of the Na2S is converted into Na2S2O3, the other half becoming NaHO. Thus oxidation of the solution is unsatisfactory in two ways.
If Russell’s sulphide is to be used to advantage, it should be preserved in hermetically sealed tanks.
It has been shown in 3 that sodium hyposulphite is obtained in most cases at less expense by allowing the sodium sulphide solution to oxidize than by buying the hypo-salt; hence, it seems timely to abolish Russell’s sulphide altogether and substitute in its place Na2S2, or even a higher polysulphide, if Na2S2 cannot be obtained free from Na2S.
All the statements made above are supported by actual experiments. I prepared from chemically pure reagents two sodium sulphide solutions, one with sufficient sulphur to contain Na2S + Na2S2, another to contain Na2S2. As I am fully aware, it is very difficult to prepare such solutions on a very small laboratory-scale, free from caustic soda or from higher polysulphides; but this does not influence in the least the principal points at issue.
The following tests were made with the freshly prepared sulphide solutions:
First, solutions of calcium chloride and of gypsum in sodium hyposulphite were prepared, of such concentration that caustic soda produced in them a precipitate of calcium hydrate.
To these solutions the sodium sulphides were now added. Each drop of the concentrated reagents produced a slight flocculent precipitate of intensely yellow color, which would disappear again upon stirring. After successive additions of the reagents, the precipitates finally remained, but to effect this, much more of the solution containing Na2S + Na2S2 was consumed than of that containing Na2S2. It became at once apparent that only a very small portion of the reagents was engaged in the reaction, the solution assuming an intensely yellow color. Upon adding a sufficient quantity of water the precipitates dissolved again; hence, it was not possible to examine them further by filtering and washing with water. This was done, however, with alcohol, in which sodium polysulphides are soluble and calcium hydrate is not. After repeated washing with alcohol the precipitates became white and proved to be calcium hydrate.
Now, 50 c.c. of each sulphide solution was put into a 7-pound acid bottle and shaken for three hours, air and carbonic acid being blown from the lungs into the bottles every few minutes. In the beginning, the solutions remained perfectly clear; finally, a small amount of sulphur separated, indicating the presence of higher polysulphides than Na2S2.
The oxidized sulphides were now tested with calcium solutions as before. Yellow precipitates appeared at once; but with equal quantities of the reagents, the precipitate from the Na2S2 solution was quite small compared with that obtained by the Na2S + Na2S2 solution. These precipitates were not flocculent, settled quickly and did not dissolve upon addition of water. After washing they became perfectly white, and proved to be CaCO3. Q,. E. D!
It is claimed that the precipitate of calcium can be avoided if precipitation of silver, copper and lead is not done too closely. I am of an entirely different opinion. If the precipitate were actually an insoluble calcium sulphide, yes; but since it is CaCO3, its precipitation must commence at once. The precipitate becomes apparent only after the metals have been completely precipitated and if calcium still remains in solution. The precipitate appears yellow, because it holds mechanically sodium sulphide. Only prolonged washing makes it white. As will be seen from the example quoted previously, much more calcium may be in a lixiviation-solution per ton of ore than can be precipitated even by a well oxidized Russell’s sulphide prepared from 6 pounds of caustic soda.
It remains to be seen how much sulphur is actually needed in practice to prepare a sodium sulphide free from Na2S. How to test such a solution for ascertaining this, has been shown above.
Regeneration of Sodium Hyposulphite From Tetrathionate
In preparing Russell’s extra-solution, one pound of sodium hyposulphite is changed to tetrathionate for each pound of copper sulphate consumed. The formation of tetrathionate is further increased by the atmospheric decomposition of extra-solution, so that, finally, the solution must hold a considerable amount of sodium tetrathionate, which salt is not a solvent for silver-compounds. Fortunately, a very simple reaction converts the tetrathionate again into hyposulphite :
Na2S4O6 + Na2Sx = 2Na2S2O3 + xS.
This reaction takes place in precipitating the solution.
So long as metals remain unprecipitated, it is doubtful whether this regeneration can take place or not. Close precipitation and over-precipitation, however, would give to the reaction free scope. Hence, it will be judicious to precipitate closely, and to over-precipitate occasionally, the solution in a number of precipitating-tanks. The excess of sodium sulphide is then neutralized by running some fresh silver solution into the precipitating-tank. That all this is not merely theoretical speculation, is proved by statistics from the Yedras mill, Mexico. With a consumption of 9.6 pounds copper sulphate per ton of ore for making extra-solution, the loss in sodium hyposulphite, by close precipitation, was only 1.4 pounds per ton of ore treated. When the consumption of copper sulphate was reduced to 7.7 pounds, the loss in hyposulphite fell to 0.74 pound. In this case over-precipitation was not practiced; if it had been done, the loss in hyposulphite would have been reduced still further. That it is profitable to regenerate hyposulphite from tetrathionate, the following calculation will show, based upon the quality and prices of chemicals assumed in 3.
For the production of 100 pounds sodium hyposulphite, a quantity of Na2S2 would be needed, requiring :

In this estimate the hyposulphite contained in and added by freshly prepared Na2S2 is included. In regenerating hyposulphite from tetrathionate, free sulphur is added to the precipitated sulphides, as follows:
For 100 parts hyposulphite regenerated, there are added:
When Na2S is used,………………………………….6.45 parts of sulphur.
When Na2S2 is used,………………………………..12.90 parts of sulphur.
When CaS5 is used,………………………………….32.25 parts of sulphur.
In these figures the hyposulphite added by and contained in the sulphides is not included.
PRECIPITATING COEFFICIENTS
The precipitating-coefficients of a sulphide solution are the quantities of silver, copper and lead precipitated for 100 parts of caustic soda, or caustic lime consumed in its manufacture. In the same way precipitating-coefficients can be established for sulphur.
If a metal is precipitated by an alkaline polysulphide, RSx, one equivalent of the latter precipitates one equivalent, or a double equivalent, of the former, (x — 1)S being liberated as free sulphur.
The following facts are of importance in reference to precipitating-coefficients for caustic soda. If the lower sodium polysulphides are prepared either from lye of proper concentration, but not of sufficient temperature before adding the sulphur, or from diluted lye by boiling it with sulphur, reagents may result with precipitating- coefficients for caustic soda far below the normal values recorded in tables A and B. This can only be explained by assuming that a part of the caustic soda remains free and uncombined with sulphur, which is actually the case. For this and other reasons already stated, it is not judicious to attempt the preparation of a solution containing Na2S in perceptible quantity in order to save sulphur. A solution in which Na2S largely predominates will always have precipitating- coefficients below the normal values for caustic soda.
On the other hand, very concentrated solutions of the higher polysulphides may give precipitating-coefficients far above normal values. Since, however, these abnormal values, as already stated, are only obtained by partial precipitation of silver, copper, and lead from a hyposulphite solution, and do not exist in complete precipitation, and consequently not in practical mill-work, the subject is without economical value, and will not be discussed here.
Although great accuracy cannot be claimed for the tables below, regarding actual mill-work, they will be sufficiently near the truth to base calculations of practical value upon them. They refer to freshly prepared sodium sulphide, and not to solutions oxidized by long contact with the atmosphere.
The following precipitating schemes may be considered of practical value.
A. Lead and calcium are absent, or only present in very small quantities.
Method 1.—Precipitation by Na2S2, or by CaS5.
B. Lead and calcium are present in perceptible quantities, or lead alone is present.
Method 2.—Precipitation of lead by caustic lime, followed with precipitation of silver and copper by CaS5.
Method 3.—Precipitation of lead and calcium by Solvay soda, followed with precipitation of silver and copper by Na2S2.
C. Calcium is present in considerable quantity, but not much lead.
Method 1, as given above, is here applicable; but in naming Na2S2 as precipitant under “C,” it is distinctly understood that the reagent should be practically free from Na2S, so that an oxidized solution contains only a minimum of Na2CO3. If it cannot be prepared with the amount of sulphur needed for the preparation of Na2S2 according to equation No. 1, more sulphur must be used.
In considering the economy of the different methods of precipitation, it would be misleading to rely only upon the figures given in the tables. There are other important elements that must enter into calculation, outside of the cost of chemicals, and the relative proportions of silver, copper, lead, and calcium in the solution. It is important, for instance, whether the lead-calcium carbonate can be profitably sold. If calcium predominates, the product may be exceedingly low in lead, and only salable at the expense of the silver it contains. An equally important factor is the grade of the sulphides in silver. The lower the grade, the heavier the freight and the smelting charges per ounce of silver, or the cost of refining if sulphides are treated at the mill. To this must be added increased expense in handling, namely, pressing, drying, sampling, and packing of precipitates. Evidently, general rules cannot be established regarding the most economical method of precipitation, but a calculation must be made for each individual case.
As repeatedly stated, tables for CaS5 cannot be calculated on merely theoretical grounds. An attempt, however, is made in tables E and F, to base values for CaS5 on actual mill-statistics.
A comparative test was made between Russell’s sulphide and CaS5, at the Cusihuiriachic mill, Mexico, as follows:
The mill, reducing 50 tons of ore per day, was run 38 days, using Russell’s sulphide, and 21 days, using CaS5 as precipitant. The quantities of chemicals consumed were as follows:
Using Russell’s Sulphide:
Average value of ore : 35.1 ounces silver per ton.
Consumption of caustic soda: 4.4 pounds per ton of ore.
Consumption of sulphur : 2.9 pounds per ton of ore.
Using CaS5:
Average Value of ore: 39.0 ounces silver per ton.
Consumption of caustic lime : 24 pounds per ton of ore.
Consumption of sulphur : 10.3 pounds per ton of ore.
Outside of a difference in value, the ore was of exactly the same character in both cases. For better comparison, I reduce the above figures for CaS5 to what they would have been in working ore of 35.1 ounces silver per ton, with the following result:
Corrected Values, Using CaS5:
Consumption of caustic lime: 21.6 pounds per ton of ore.
Consumption of sulphur: 9.3 pounds per ton of ore.
To make these statistics more available for comparison, the composition of the sulphides should have been ascertained, besides the consumption in hyposulphite. Under the circumstances, I have to take the figures as they are. Considering what has been said about the preparation of CaS5, and its deterioration by oxidation, the much higher consumption of chemicals involved in manufacturing CaS5, compared with Russell’s sulphide of equal efficiency, is by no means extravagant. To make comparison perfectly fair, we should add, however, for CaS5 a gain of about 20 per cent, in hyposulphite, on a basis of the quantity of this salt contained in freshly prepared Russell’s sulphide. For convenience in calculation, the addition of calcium hyposulphite is put down as its equivalent of the sodium salt.
In the financial table F, the price of caustic lime is taken at ¾ cent per pound, and other chemicals as stated in table D.
It is interesting to compare table F with table D. Even under favorable assumptions for CaS5, this reagent is, at stated prices for caustic soda, sulphur and sodium hyposulphite, very much dearer than Na2S2.
CaS5 would be only cheaper where caustic lime can be obtained at much less than ¾ cent per pound, and where the cost of sulphur is abnormally low compared with that of caustic soda.
For this reason, in examining different methods of precipitation for a special case under normal conditions, we may just as well pay no attention to CaS5 in Method 1, and discard Method 2 altogether.
Finally, if in lixiviation a considerable amount of sodium hyposulphite is consumed to keep up the strength of the stock-solution, it will be more profitable, in most cases, to use an oxidized sodium sulphide solution in place of one freshly prepared. Although the cost of precipitation is thereby apparently increased, the total cost of lixiviation will be diminished.
It has been claimed that in using CaS5, the sulphides precipitate quicker and settle better than with sodium sulphide. This claim is not sustained by practical experience.
We find it stated in treatises on metallurgy that calcium hyposulphite is a more energetic solvent for gold than the sodium salt. This statement is without foundation in theory or practice. The solvent energy of calcium hyposulphite for silver-compounds is slightly inferior to that of the sodium salt, according to Russell’s experiments.
The difference in deterioration of the two hyposulphites by atmospheric influences has already been mentioned.
TABLE A
Precipitating-Coefficients for c. p. Reagents
Calculations are based upon the following approximate chemical equivalents, frequently used:
H = 1; O = 16; C = 12; S=32; Na = 23;
Ca = 40 ; Ag= 108; Pb = 207; Cu = 63.
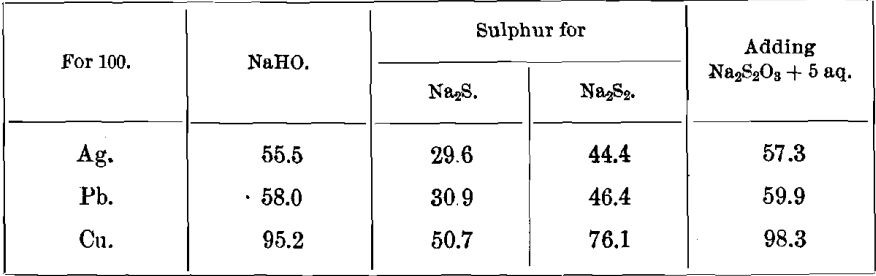
Precipitating-Coefficients for NaHO, Consumed in the Manufacture of Na2Sx.
100 NaHO precipitates 180.0 Ag as Ag2S.
100 NaHO precipitates 172.5 Pb as PbS.
100 NaHO precipitates 105.0 Cu as Cu2S.
Adding 103.3 Na2S2O3 + 5 aq.
The following quantities of NaHO and S, consumed in the manufacture of Na2S and Na2S2, are needed for the precipitation of metals:
Precipitation of Lead and Calcium by Na2CO3
100 Na2CO3 precipitates 195.3 Pb as PbCO3.
100 Na2CO3 precipitates 37.7 Ca as CaCO3.
For precipitation of
100 Pb are needed 51.2 Na2CO3.
100 Ca are needed 265.0 Na2CO3.
TABLE B
Precipitating-Coefficients far Commercial Reagents
Caustic soda with 90 per cent. NaHO, corresponding to the English rating of 70.6 per cent.; sulphur with 95 per cent. S ; Solvay soda with 98 per cent. Na2CO3.
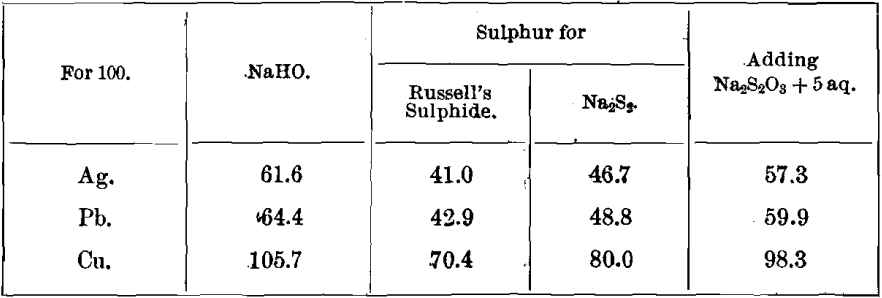
Precipitating-Coefficients for Caustic Soda, Consumed in the Manufacture of Na2Sx
100 Caustic soda precipitates 162.0 Ag as Ag2S.
100 Caustic soda precipitates 155.2 Pb as PbS.
100 Caustic soda precipitates 94.5 Cu as Cu2S.
Adding 93 Na2S2O3 + 5 aq.
The following quantities of caustic soda and sulphur, consumed in the manufacture of Russell’s sulphide and Na2S2, are needed in the precipitation of metals :
Precipitation of Lead and Calcium by Solvay Soda
100 Solvay soda precipitates 191.4 Pb as PbCO3.
100 Solvay soda precipitates 36.9 Ca as CaCO3.
For precipitation of
100 Pb are needed 52.2 Solvay soda.
100 Ca are needed 270.3 Solvay soda.
TABLE C
Weights of Precipitates and their Percentages in Metals
Weights of Sulphides
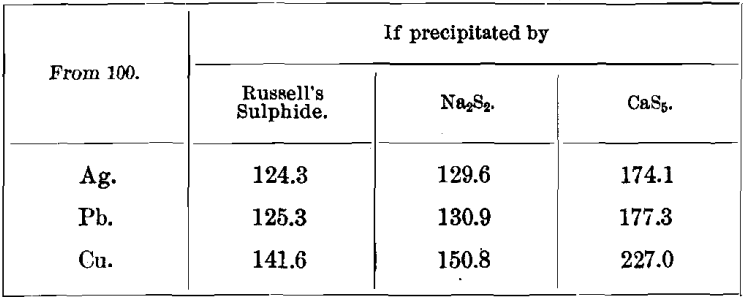
Weights of Carbonates.
The precipitation by Solvay soda will produce from:
100 Pb,…………………………………………………………………..129.0 PbCO3.
100 Ca,…………………………………………………………………..250.0 CaCO3.
Percentages of Metals in Sulphides
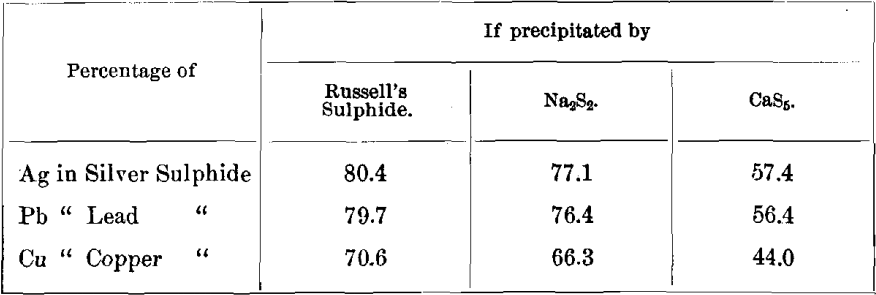
Percentages, of Metals in Carbonates
Lead carbonate, 77.5 Pb.
Calcium carbonate, 40.0 Ca.
TABLE D
Financial Results
Showing the cost of precipitation by Russell’s sulphide, Na2S2, and by Solvay soda, assuming the following prices for commercial chemicals put down at the mill: caustic soda, 5.5 cents; sulphur, 3.5 cents; Solvay soda, 4.0 cents; hyposulphite, 4.0 cents per pound.
Cost of Precipitating Metals as Sulphides
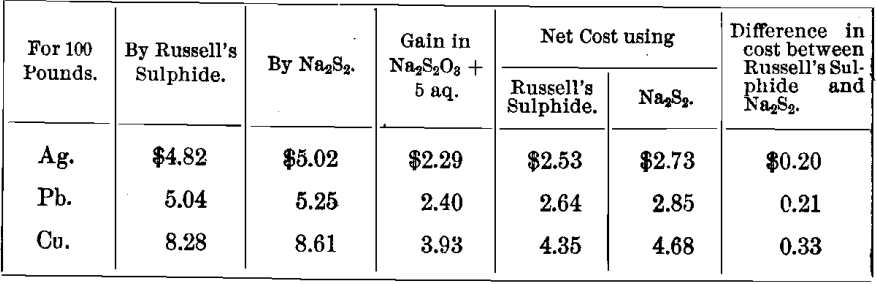
Cost of Precipitating Lead and Calcium as Carbonates
For 100 pounds Pb, $2.09.
For 100 pounds Ca, 10.81.
TABLE E.—Showing the quantities of caustic lime and sulphur consumed in the manufacture of CaS5, needed for the precipitation of metals.
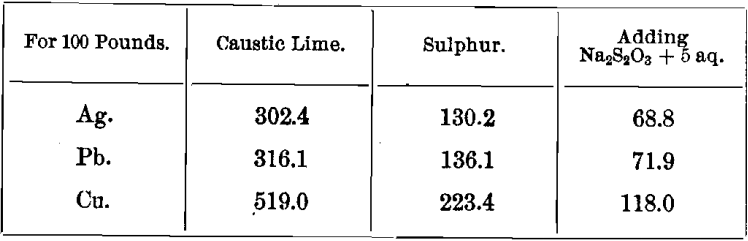
This table is based on comparative statistics obtained in the Cusihuiriachic mill, Mexico.
TABLE F.—Showing the cost of precipitation by CaS5, assuming price of caustic lime at ¾ cent per pound, and that of other chemicals the same as given in table D.
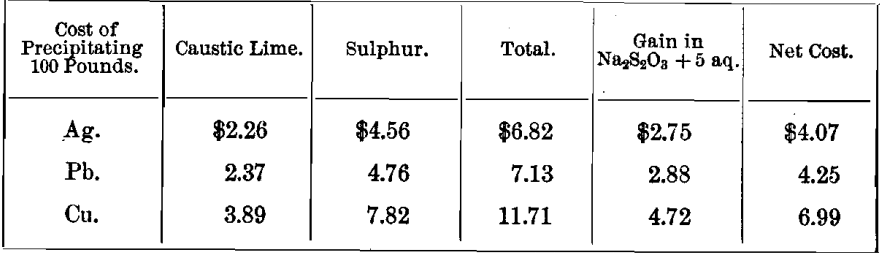
This table is based upon the figures given in table E.
Application of the Tables to Special Cases
In this section the tables will be applied to examine the cost of precipitation in special cases. It is assumed that the ore contains lead and a small amount of calcium. Although its contents in copper are insignificant, the copper from extra-solution, necessary for treatment, comes to precipitation. We will neglect the comparatively small amount of silver precipitated with the lead-calcium carbonates, assuming that such silver is paid for at the same rates as silver in sulphides. Prices of chemicals are taken as stated in Table D.
We further assume the following terms, according to a case as it really exists, for the disposal of the sulphides to smelting-works and the cost of handling the precipitates:
The Marsac mill, according to W. A. Wilson, sells its sulphides to the Omaha & Grant S. & R. Co., the latter paying for 97 per cent, of the silver, New York quotations, charging $30 per ton for treatment, but paying freight from Park City to Omaha. No allowance is made for copper or lead. [Gold is paid for at the rate of $20 per ounce.]
Carbonate precipitates are taken at the same rates, as also the low- grade precipitates from the first wash-water. It will be seen at once that the disposal of carbonates at sulphide rates cannot be favorable to the separate precipitation of lead in the presence of calcium. The Marsac mill used this method formerly for a short time only, then abandoned it, but has adopted it again recently. A better market for carbonates will, no doubt, be found hereafter.
The cost of handling precipitates is assumed at $10 per ton.
EXAMPLE
The solution obtained in lixiviation contains per ton of ore :
3 pounds (43¾ ounces) silver.
1 pound copper.
3 pounds lead.
½ pound calcium.
Four pounds sodium hyposulphite have to be regenerated from tetrathionate in precipitation. This expense, however, we need not consider, since it is the same for Methods 1 and 3.
I hardly need remind the reader that in precipitation Na2S2 free from Na2CO3 is used, and not an oxidized solution of Russell’s sulphide, which would also precipitate calcium in Method 1, changing the weight and grade of the sulphides.
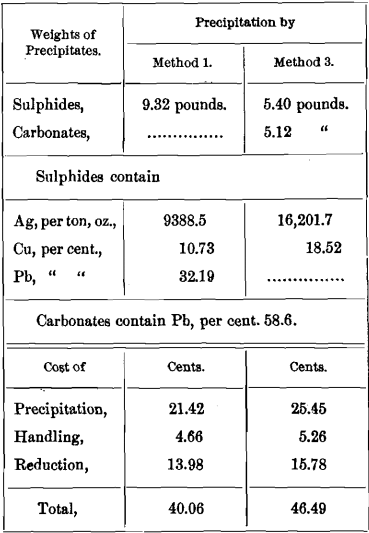
It will be seen at once that separate precipitation of lead and calcium cannot be profitable if carbonates are sold at sulphide rates. Also, that Method 3 becomes more costly, the more calcium is present in proportion to the lead.
We will now assume 90 per cent, of the lead in carbonates to be paid for at the rate of 2 cents per pound, and freight and smelting- charges to be $15 per ton; also that silver is paid for at sulphide- rates. This would give the following result:
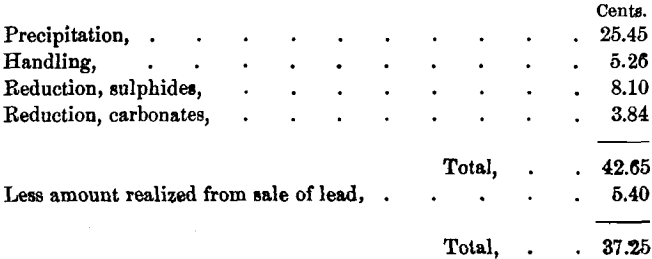
Should the buyer, however, deduct 5 per cent, of the silver in place of 3 per cent., nearly all the profit from the sale of lead may be consumed, or even a negative balance left, depending on the richness of the carbonates in silver.
It is hardly necessary to continue this discussion. The separate precipitation of lead and calcium by Solvay soda is evidently not so profitable as its advocates claim. If lead alone is present, or the amount of calcium is very slight in proportion to lead, Method 3 can be used to advantage.
It is claimed that the removal of calcium from the lixiviation-solution has a beneficial effect on the extraction of the silver. Whether this is true, I do not know.
If sulphides are refined at the mill by a humid process with sulphuric acid, the absence of lead and calcium is essential to success; and in this case Method 3 is of great value, as I shall demonstrate in another paper.
My friends who operate lixiviation-works will, most likely, object to many conclusions I have drawn in this treatise, saying that the process as carried out in practice is not like that operated here on paper. If they will bring out an array of facts, proving that I am wrong, I shall consider myself the gainer, earning the thanks of the profession for bringing information to light which otherwise would have remained hidden.
APPENDIX
The Loss of Hyposulphite in Lixiviation
In connection with precipitation, some remarks about the loss of hyposulphite in lixiviation will be pertinent.
As has been shown, large amounts of hyposulphite are added to the stock-solution in precipitation, even with freshly prepared calcium or sodium sulphides. From this it might be expected that the stock-solution would always increase in strength and concentration. This is only the case if, in ordinary lixiviation, CaS5 is used as precipitant, and comparatively large amounts of copper and lead are thrown down together with the silver.
The losses in hyposulphite are partly mechanical, and partly caused by chemical decomposition. The mechanical losses take place when the first wash-water is replaced by stock-solution, and again when the latter is replaced by the second wash-water. In these operations, no matter how carefully they are conducted, water and stock-solution are more or less mixed, especially where the filters are not in good condition and are partly choked. Thus, in replacing the first wash-water, a certain quantity of hyposulphite solution results, too weak to be mixed with the normal solution; for this reason it is transferred to a separate precipitating-tank, precipitated there by itself, and the decanted clear solution run to waste. As soon as the solution shows a certain strength in hyposulphite, which is ascertained by the iodine-test, it is turned into a regular precipitating-tank. The same is the case in replacing the solution by the second wash-water. The higher the percentage of hyposulphite in the stock-solution, the greater this mechanical loss.
The chemical losses are caused as follows:
- By oxidation in contact with the atmosphere, hyposulphite being converted into sulphate. This loss depends on the quantity of solution used per ton of ore and kept in rotation, its temperature, and upon atmospheric conditions, most likely the contents of the atmosphere in ozone.
- By converting hyposulphite into tetrathionate in preparing extra-solution, and further formation of tetrathionate by atmospheric oxidation of extra-solution. This loss is, however, made largely good again by close precipitation, and may be almost completely covered by over-precipitation.
- By decomposition of extra-solution with formation of Cu2S and sulphates ; this loss being final and irretrievable. It is greatest where extra-solution has to perform much work—i.e., where standard extra-solution must be used warm and is circulated. It is, no doubt, greater if a Koerting ejector is used for circulation than with a geyser-pump. This subject is discussed in my paper, “ The Construction of Details for a Modern Lixiviation Plant.” (Page 3 of this volume.)
