The occurrence of the tellurides of gold and silver, even in small quantities, is so rare that their metallurgical treatment has engaged little attention. A residence of several months at one of the few mines in North America the product of which consists entirely of such ore, has led me to arrange a method for its economical treatment. The mine referred to is the Huronian, situated a few miles distant from Jackfish Lake, in Ontario, Canada. The plan proposed is for this peculiar case, and is put on record as possibly suggestive and helpful in any similar instance, should it occur. Since freight-rates from the mine to the nearest railroad-point were extremely high, and for other economical reasons, the method proposed is modified by the use, as far as possible, of the machinery already at the mine, and is so arranged that bullion instead of concentrates would be obtained as a final product.
The ore-vein at the Huronian mine varied in width from about 4 feet at the surface to 2 feet and less at the lowest level—125 feet —and dipped southeast about 88°. The rock consisted, on both foot- and hanging-walls, of soft, green Huronian slates carrying much highly crystalline but barren pyrite. The ore carried an inconsiderable amount of galena with iron and copper pyrites, argentite, hessite, sylvanite (or petzite), and probably altaite, the telluride of lead. Near the surface the ore showed considerable native gold, but this disappeared with greater depth. Two complete analyses of minerals containing tellurium are here attached, as they establish the presence of two important compounds:
I. Analysis of a mineral supposed at the mine to be sylvanite, but agreeing more closely in composition with petzite (Ag, Au)2 Te:
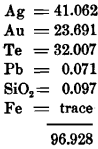
The specific gravity of this mineral was 8.103 and the color was steel-gray.
II. Analysis of a telluride of silver:

The sample was probably hessite (Ag2 Te), in which some of the tellurium was probably lost in the analysis.
In some cases the tellurides existed in irregular, bluish-gray masses enclosed in the pure quartz; at other times they occurred as very dark brown scales, filling small seams in the quartz. Samples of quartz from this mine, which showed no trace of metallic contents, but seemed to be saturated with the tellurides and resembled an ordinary piece of smoky quartz, have given upon assay as high as 902 ounces of silver and 34 ounces of gold per ton. Through the kindness of the manager of the Huronian mine, Mr. Thomas A. Keefer, of Port Arthur, Ontario, I am permitted to publish the figures obtained in an experimental run of one week upon pure vein- rock from the mine. The values given to the various products are probably all too high, owing to the lack of a proper assay-balance, but, as they are approximate and not too low, they will suffice for the purpose.
The ore was crushed, wet, in two five-stamp batteries, amalgamated on copper plates, and concentrated upon three Frue vanners, each vanner being provided with an amalgamated copper plate on its distributor. The ore crushed in 5½ days, including night run, amounted to 46.7 tons, or 93,400 pounds, its assay value per ton being $57.13, or its total value $2667.
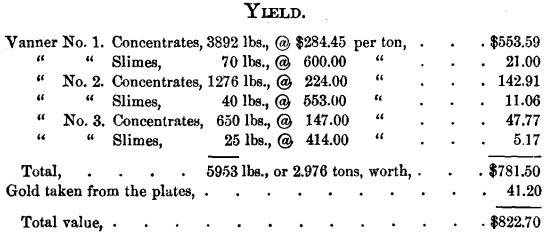
Loss, 69.1 per cent, of ore-value.
Average value of concentrates per ton, $262.60.
Yield per ton of crude ore treated, $17.61.
Concentrates represent, in bulk, 6.35 per cent, of the crude ore treated ; in value, 29.3 per cent.
Work per day (24 hours) of ten stamps, 8.49 tons.
An explanation of the extraordinary loss in the above test-run is not difficult. The machinery used was purchased at second-hand from an abandoned enterprise near Port Arthur, with the hope, on the part of the management, that it would suffice to treat the ores of the Huronian mine, the peculiar character of which was not understood. The ore treated came from some distance below the surface, and carried but little free gold, as is shown by the value of the amalgam saved from the coppers—something less than $1 per ton of ore treated. The tellurides of gold and silver are extremely sectile, and this property alone is sufficient to render futile the method used, since much of the valuable part of the ore could not be saved by the Frue vanners, either in the concentrates or in the slimes, but floated off with the water and tailings as an impalpable powder. This is proved by the comparatively high value of the slimes, or that part of the pulverized ore which was not washed off the belt, but which was still light enough, when disengaged from the heavier ore-particles, to float upon the surface of the water, and thus pass into the slime-boxes.
It is evident that the method employed for treating the ores in question was decidedly the wrong one in view of the yield obtained. But even if the concentrates could have been made to contain the full assay value of the ore, deducting the gold found on the coppers, the difficulty of transportation to shipping-point made it a mistake to aim at producing anything but bullion.
Although the precious-metal contents of these ores might be saved by a smelting with lead, the cost of obtaining enough lead to supplement the very small percentage in the ore, the uncertainty whether the extent of the ore-deposit, when thoroughly explored, would warrant so extensive and costly a plant, and the uncertainty as to the existence of any neighboring deposits of smelting-ores, rendered the method inapplicable in this case.
The processes used in the extraction, in the wet way, of the precious metals from their sulphuretted ores generally make use of a primary roasting, either with or without fluxes. Since the telluride of gold is volatile at and below a roasting-heat, the most carefully arranged dust-chambers would scarcely avoid a very large loss. It is probable that no process of amalgamation could be applied with good results to these ores, at least when raw, since mercury attacks the gold of tellurides only after prolonged digestion, and then only to a limited degree. It may be said that tellurides do not amalgamate.
We find ourselves subject to loss of precious metals in two principal operations, viz., concentrating and roasting, and, at the same time, are limited in our choice of a method to some wet process. As the ore is of too low a grade to treat as a whole, it must be concentrated. Therefore, a plan is suggested to modify the mode of concentration used so as to reduce the loss to a minimum.
Thus far the saving of the gold and silver only has been considered, but there is still a valuable metal in these ores, i.e., the tellurium, of which Mr. T. A. Edison says, in a private communication to the writer: “ I have worked it to a considerable extent, and I pronounce it a marvellous substance; I could use it in large quantities if it were cheaply produced.” Its great cost and its scarcity have, doubtless, retarded the knowledge of its valuable properties. About forty years ago Wohler wrote largely and enthusiastically upon its organic compounds and its pharmaceutical uses. It possesses properties similar to those of selenium, which changes its electrical resistance under the influence of light. It may then be advisable to attempt to save the tellurium.
As already stated, no attempt is made to decide upon the best method for treating the ores in general which carry tellurium, or even to decide upon the best method, in the abstract, for treating ores peculiar to this mine; but rather, for various economical reasons, to make use, in part at least, of the apparatus already at the mine by supplying necessary modifications, and finally to produce fine bullion.
Various methods of treatment of the tellurides of gold and silver have been suggested and actually used, a few of which are referred to. With the exception of the last two, however, they contain evident faults, or would for other reasons be unfitted for use in this case.
(1.) In Transylvania, the tellurium has been recovered by fusion of the pulverized ores with sodium carbonate and subsequent separation from the soluble sodium telluride. The other metals were afterwards extracted from the residue.
(2.) Boulder County, Colo., has produced large quantities of tellurides. In the neighborhood of Gilman such ores are found in flat veins in the quartzite. These ores are either mixed with others and smelted with lead or are sent to the Boston and Colorado smelter at Argo where everything goes into a copper-matte.
(3.) Some years ago a method for the treatment of tellurides was recommended by Mr. C. A. Stetefeldt. The ore was to be crushed and passed through rolls, then screened and three products obtained of which the first or coarsest was to be concentrated in jigs, the second or finer product was to be concentrated on joggling tables and the third product or fines was to be concentrated upon rotary buddies. Of the three products of concentration the richest was to be cupelled with lead—the ore being added gradually. The tellurium, according to Mr. Stetefeldt, would partially volatilize and in part become PbTe and form “ abstrich ” with the PbO. When this abstrich ceased to form, the auriferous and argentiferous lead was to be drawn off and cupelled upon another test. The abstrich was to be melted in a reverberatory furnace, to remove PbO, and the residue from this process worked over, to save the tellurium.
In Mr. Stetefeldt’s process the proposed concentration of the fines upon round buddies would undoubtedly prove a fatal defect.
(4.) The Walker-Carter vapor-amalgamation process is said, by Mr. E. N. Riotte, in a private communication to the writer, to be capable of saving the gold and silver from tellurides.
(5 and 6). Finally, the Hauch process has been successfully used, as has also the process described by Schroder, the latter with the tellurides of Nagyag, Transylvania; and an adaptation of these methods is proposed for the treatment of the ores of the Huronian mine.
The accompanying drawing gives an arrangement of the apparatus proposed for the separation of the fine tellurides and the concentration of the coarse remainder. The dry ore is broken in the crusher,
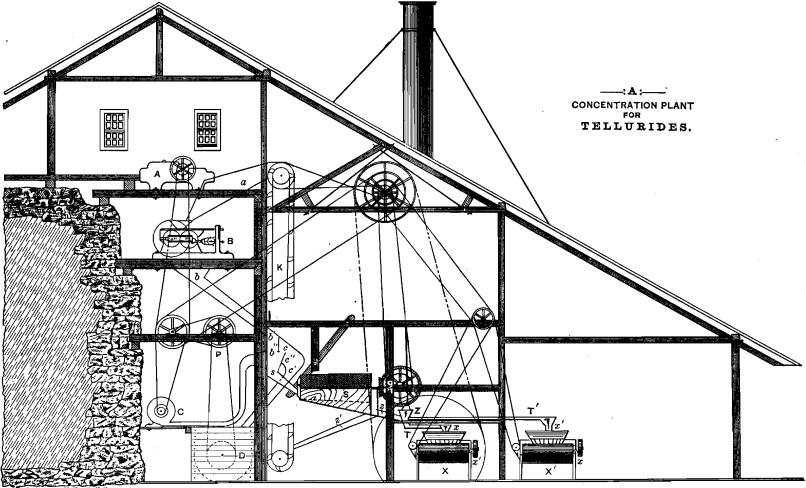
A, and passes thence, through the chute, a (which is carefully closed in to prevent loss of the fines), to Cornish rolls, B. From the latter it slides in the covered chute, b, but, on falling vertically from the point, b’, the stream of ore meets, at the point b”, a blast of air from the blower, C. The dust from the pulverized ore is blown through the opening c, and falls into the chute, c’. It would seem advisable that the air-blast be so regulated as to deflect and throw against the inclined screen, c”, all of the fines which are yet too heavy to be blown over it through c. The screen, c”, may be made of 40- to 50-mesh wire-gauze, and the portion of fines passing through it joins the dust in chute, o’, and passes with it into the tank, D. This tank may be lined with lead and filled with a solution of dilute HCl to such a level that the nose of the chute, o’, dips just beneath it. This arrangement causes the blast of air to perform the additional service of partially agitating the solution of HCl. The latter, being an absorbent medium for the fines, has the further function of preparing this part of the ore for the ensuing operations. The tank, D, should be provided with a cylindrical bottom in which any convenient form of stirring apparatus may be caused to revolve by the pulley, P. It should be provided also with a proper outlet for the withdrawal of the pulp.
The portion of the ore which has resisted the deflecting action of the air-blast continues on its way and passes through the chute, s, into the revolving wire-cloth screen, S. Its mesh must be determined experimentally.
The ore passing through it goes through the chute, z, into the sizer, Z. The coarser portion which does not pass through the screen is conducted by the chute, z’, to the conveyer, K, which returns it to the rolls, B.
Water is admitted to the sizing apparatus through the cock, W, and the two sizes of pulverized ore pass through the troughs, T and T’, respectively, and are deposited upon the distributors, x and x’, of the Frue vanners, X and X’.
By the above arrangement only the ore-particles large enough to escape the blast of air, or of a size greater than 40- to 50-mesh, reach the vanners. They are all too large and heavy to lead to serious loss in concentration.
The particular point at which the blast of air is introduced, its pressure, and the choice of the most convenient size and weight of screens, are all matters to be regulated by trial. The concentration should be so conducted that the concentrates and fines together should represent not over 10 per cent, of the ore treated.
From a knowledge of the character of the ores it is believed that, after being, crushed dry as in this case, the greater portion of the sectile tellurides will be collected as dust in the bin, D. If this be the case we may treat the concentrates from the vanners by one of the following methods :
1st. Roast, if the remaining amount of tellurides is not found to be too great; pass through the stamps and amalgamate, or treat by such method as the character of the valuable mineral-content renders advisable.
2d. Roasted or raw, as may be found best on trial, the concentrates may be finely pulverized and treated with the dust from bin, D.
The fines, after withdrawal from bin, D, in the form of pulp, may be agitated and mixed together in a heated solution of dilute HCl, this drawn off and the pulp further agitated with hot, concentrated HCl until no more H2S escapes, the operation being performed in a lead-lined vessel or tank.
Chlorine may be generated directly in this solution by use of oxide of manganese, or the solution of concentrated HCl may be drawn off for use with the next charge of fines, and the pulp treated with aqua-regia. The tellurides are dissolved with the other minerals, the solution is drawn off and the residue washed. The lead and lime may be removed from this solution by a small addition of H2SO4. The solution is then decanted from the precipitate and the gold thrown down by means of FeSO4. After filtration the tellurium may be precipitated with metallic zinc and purified by treatment with chlorine and H2SO4.
The chloride of silver left in the pulp-residue may be decomposed by means of zinc and HCl, the metallic silver dissolved by H2SO4 and re-precipitated from the sulphate solution by means of metallic copper.
The process above outlined seems to do away with the loss in concentration and also with a part of the loss in roasting. If much of the tellurides should remain in the concentrated portion of the ore, the roasting ought to be avoided if possible; for a series of small muffle tests upon a rich sample of telluride-bearing quartz satisfactorily proved the inevitability of loss by volatilization. The experiments were conducted at various heats, with and without salt, and in every roasting loss occurred, commencing even before the sample became red. Where the loss by volatilization is so very great it seems dangerous and expensive to trust entirely to condensing-chambers. No experiments were made to show the loss of gold in roasting the tellurides with admission of superheated steam. In laboratory-experiments with the same ore, the gold, silver and tellurium were separated without difficulty by means of the method described.
