Viscosity measurement represents one of the simplest experimental techniques for characterizing polymers. In general, the viscosity of a polymer solution depends on the molecular weight of the polymer and on the configuration of the molecules. For essentially linear polymers such as the polyacrylamide flocculants, coiling of the molecule in response to changes in its environment leads to changes in viscosity which can be evaluated in terms of an effective radius of gyration.
For flexible molecules which form random coils in solution it can be shown that the radius of gyration (Rg), defined as the root-mean-square distance of the elements of the chain from its center of gravity, can be calculated from viscosity data using the following relation:
[n] = 14.7∅Rg/M………………………………………………(1)
where M is the molecular weight of the polymer, ∅ is Flory’s universal constant (2.1×10)21) and [n] is the intrinsic viscosity of the solution defined by:
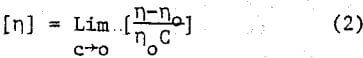
in which n is the viscosity of a polymer solution of concentration C and η0 is the viscosity of the pure solvent.
It should be emphasized that the application of Equation 1 to aqueous solutions of polymer flocculants is approximate at best since it is by no means clear that such molecules do indeed form random coils. However, it is probably reasonable to assume that the values of Rg calculated for a given polymer under different conditions reflect the changes in molecular configuration which occur in response to changes in environment.
Figure 1 is a typical plot for polymer A, under various conditions. The variations in the rheological properties of these dilute solutions can be attributed to three electroviscous – effects of which the change in the polymer configuration due.to ionization of carboxyl groups is the most important. Although all three polymers are listed as non-ionic by the manufacturer they behave differently. For example, there is a five-fold increase in the intrinsic viscosity of polymer A when its solution is acidified to a pH of 3.5 while only slight changes are observed for polymers B and C within the same pH range.
In light of these results one can conclude that polymer A is slightly hydrolyzed while the other two are essentially non-ionic polyacrylamide. Thus at low pH, where the dissociation of carboxyl groups of polymer A is reduced, the polymer chain, which under these conditions carries very little charge, will coil up and cause a reduction in, the intrinsic viscosity.
Under the same conditions the chains of polymers B and C will undergo only slight changes since there are very few (if any) carboxyl groups to react with the hydrogen ions. At very high pH, the intrinsic viscosity of the polymers tends to decrease because of the distortion of the ionic atmosphere around the polymer chain as well as changes in the effective net charge on the polymer. Decrease in intrinsic viscosity was observed for all of the polymers upon addition of sodium chloride, albeit to different extents.
The response to ionic strength seems to indicate that all the polymers carry some negative charge. This has been confirmed by electrophoretic mobility measurements on various solids onto which polymers A, B, and C had been adsorbed. The results showed that the particles coated with polymer A always carry a higher negative charge than those coated with polymers B or C.

