Alkali metal ferrocyanides and their easily obtainable derivatives such as ferricyanides, nitroprussides, and hydroferrocyanic acid have found application in a wide variety of fields during the two centuries that they have been known. Through continued research new uses are being uncovered with increasing frequency.
Most of these applications depend upon oxidation-reduction potentials, sequestering and precipitating ability, color formation, or the high nitrogen content of the prussiates. In the following sections, the applicability of these properties to a number of specific uses will be discussed.
ANALYTICAL CHEMISTRY
Since ferrocyanides and their derivatives have found so many and such diverse applications in analytical chemistry, a complete coverage of the field is beyond the scope of this review. The following, however, will indicate some of the potential uses of these compounds as analytical reagents.
Potassium ferrocyanide has been found useful for the qualitative detection of certain sugars, the nitrite ion in urine, thiourea, a number of alkaloids, and various metals such as cadmium, iron, uranium, and zinc.
The quantitative determination of zinc, cadmium, cerous ion, uranyl ion, and lead also may be accomplished through the use of potassium ferrocyanide. The same salt has been reported in a new analytical method for leaded zinc oxides. Lithium ferrocyanide in an ethanol-water solution quantitatively precipitates both sodium and potassium ions. If potassium is to be determined separately, however, a mixture of water and ethanol containing sodium ferrocyanide as well as calcium chloride may be used. The ferrocyanide ion is the basis of an indirect colorimetric method which has been used for the determination of potassium in blood.
α-Naphthol and β-naphthol form differently colored solutions with potassium ferrocyanide, and their differentiation may be accomplished by this means. The separation and identification of numerous alkaloids and other organic bases are discussed more extensively in another section (p. 80).
Pentacyanoammineferroates [M3Fe(CN)5NH3] and nitroprusside are reported to be useful in qualitative analysis. Sodium nitroprusside, for example, aids in the detection of sulfite ion and several alkaloids as well as in the qualitative and quantitative determination of thioesters. 2-Carbethoxyethyl thioacetate and acetyl-2-aminoethyl thioacetate are among the compounds that form deep red-violet colors with sodium nitroprusside.
BLUEPRINTS
Blueprint paper is coated with a mixture of a ferric salt of an organic acid (ferric ammonium citrate, etc.) and potassium ferricyanide. A portion of the ferricyanide may be replaced by potassium ferrocyanide to produce a coated paper that has greater printing speed but yields a print with satisfactory latitude. When these treated papers are acted on by strong light through a pattern, blue ferrous ferricyanide is formed in the exposed areas. The unexposed salts are removed later by washing. Sodium diammonium ferricyanide, Na(NH4)2Fe(CN)6, prepared easily by the electrolytic oxidation of sodium ferrocyanide followed by reaction with ammonium sulfate, is said to be a good substitute for potassium ferricyanide in this application.
CASE HARDENING AND HEAT TREATING
Mixtures containing potassium ferrocyanide are useful in the carburizing and heat treating of files, saws, and knurled parts. One suggested formulation contains by dry measure one part of potassium ferrocyanide, one part of sodium chloride, and one-half part of rosin, powdered and intimately mixed. The steel part is warmed and rolled in this mixture, which melts and adheres. The coated part is packed in sawdust, earth, or other inert material within a box, and the entire assembly is then fired at 1600-1700 °F. A period of four to twelve hours is required to case-carburize the steel, which is further treated by quenching and tempering. Steel files may be coated with other compositions containing ferrocyanides prior to heat treatment in molten lead, thereby minimizing the amount of lead that adheres to the surface and reducing decarburization of the steel.
To develop a light case on steel tools, they may be heated in a torch to cherry red, dipped in powdered potassium ferrocyanide and reheated. This process may be repeated if necessary to secure greater depth of penetration. Following the final reheating, the parts are quenched in oil or water as desired.
CHEMICAL SYNTHESIS
Catalysis
Ferrocyanides, or products readily obtainable from them, have been used as catalysts for numerous chemical syntheses. According to an early German patent, the residue from the pyrolysis of potassium ferrocyanide at 600-700 °C. serves as a catalyst for the synthesis of ammonia from a nitrogen-hydrogen mixture under pressure at 430°C. Pyrolyzed alkali metal ferrocyanides have been used in this country in the same synthesis. A pyrolyzed mixture of sodium or potassium ferrocyanide combined with calcium or magnesium ferrocyanide has been reported as being effective in the synthesis of cyanogen from natural gas and air. Catalysts for these reactions having greater surface area may be prepared by absorption of the soluble ferrocyanides on inert materials followed by pyrolysis under specified conditions. The double salt, potassium aluminum ferrocyanide, when pyrolyzed, also is reported to be active as an ammonia-synthesis catalyst.
In the electrolytic preparation of potassium persulfate, the addition of potassium ferrocyanide to the bath produces a 20-23 percent increase in yield, based on the current used.
Various ferrocyanides and their derivatives, such as potassium ferrocyanide, potassium ferricyanide, tetraethyl ferrocyanide, and sodium nitroprusside, are said to accelerate the absorption of olefins by sulfuric or phosphoric acid, thereby aiding the production of alcohols, esters, and ethers. Some undesirable side reactions are eliminated by this means.
A salt mixture containing potassium ferrocyanide, aluminum chloride, and ferric chloride has been used as a catalyst to promote the addition of halogens to olefinic hydrocarbons at temperatures in the range 80-250°C. The catalyst is prepared by evaporation of an aqueous solution containing the salts.
Chemical Intermediates
When treated with hot concentrated mineral acids, sodum and potassium ferrocyanides slowly evolve hydrogen cyanide. Utilization of this decomposition in the manufacture of pure alkali cyanides has been suggested, but such a process does not appear to be economically feasible.
Alkali cyanides also are prepared in good yield from the fusion of potassium or sodium ferrocyanide with the respective alkali metal.
Oxidation-Reduction Reactions
Control of chemical reactions involving oxidation or reduction may be improved by the selection of agents which have the desired oxidation-reduction potentials. The ferrocyanide-ferricyanide system has found application in many different reactions of this type, some of which are outlined in this section. It is notable that the products often have considerable therapeutic interest. A more complete discussion of the chemical reactions involved may be fund on pp. 34 ff.
Studies of the catalytic oxidation of linseed oil in the presence of potassium ferricyanide led to the conclusion that the rate of oxygen absorption is dependent upon the concentration of the oxidized form of the catalyst to the two-thirds power and is inversely proportional to that of the reduced form to the one-third power. On the basis of this investigation, the following equation was formulated:
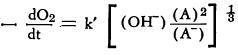
(A) = concentration of potassium ferricyanide and (A-) concentration of potassium ferrocyanide. The average values of k’ at 25.0°C. were found to be 4.1 to 4.8 x 10-2.
Certain tertiary methylamines may be demethylated through the use of alkaline ferricyanides if the methyl or dimethylamino group is attached to a secondary or tertiary carbon atom.
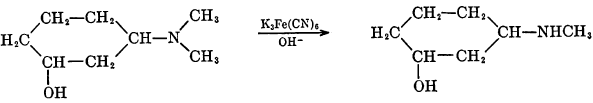
During this reaction, the mechanism of which has not been elucidated, the ferricyanide ion is reduced.
Ammoniacal cuprous chloride reacts with 5-methoxy-1-pentyne to form the cuprous salt. When treated with concentrated potassium ferricyanide solution, the cuprous salt is converted in good yield to 1,10-dimethoxy-4,6-decadiyne.
![]()
Potassium ferricyanide oxidizes o-phenylthioacetanilide to form 2-methyl-4-phenylbenzothiazole.
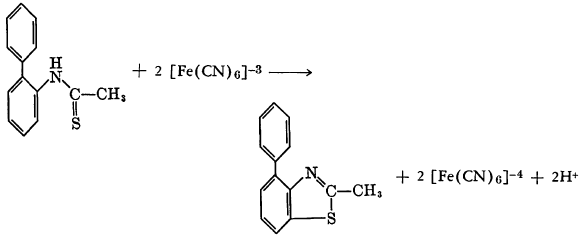
An analogous reaction has been reported to occur with potassium ferrocyanide rather than the ferricyanide. In this case N-thioacetyl-m-anisidine in 4 percent sodium hydroxide was added to potassium ferrocyanide in water with stirring.
From the reaction mixture 5-methoxy- and 7-methoxy-2-methylbenzothiazoles were isolated as their picrates (p. 62).
Adrenalin, dissolved in water as the acetate, has been treated with potassium ferricyanide and sodium bicarbonate, followed by sodium hydroxide. After acidification with acetic acid and standing, 3,5,6-trihydroxy-1-methylindole separated as the hydrate.
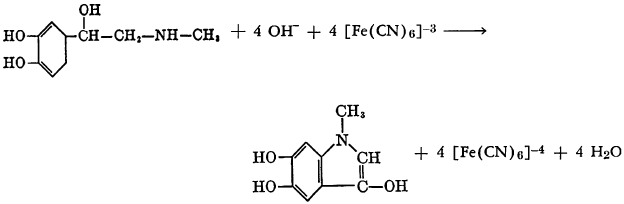
N-methylnicotinic acid undergoes hydration and oxidation in aqueous solution in the presence of potassium ferricyanide to yield 1,6-dihydro-1- methyl-6-oxonicotinic acid. The reactions may be formulated as follows:
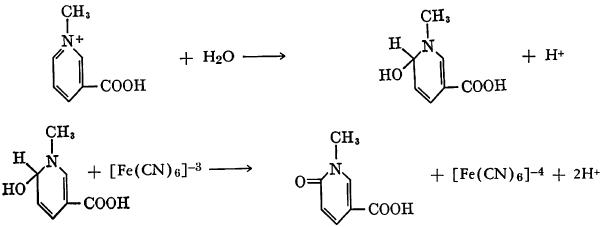
Isoquinoline may be N-methylated and then treated with alkaline ferricyanide solution. The probable intermediate, N-methyl-1-hydroxy-1,2-dihydro-isoquinoline, is then oxidized by the ferricyanide to 2-methyl-1,2-dihydroisoquinolone.
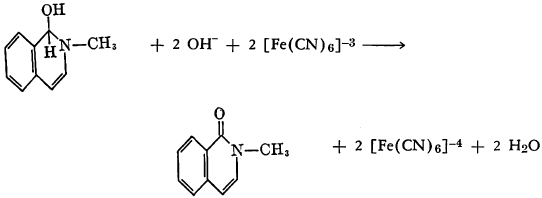
Several aromatic diazo compounds were found to react with potassium ferrocyanide to yield arylazodiaryls, the reaction proceeding most smoothly at pH 4 to 5 and in the presence of sodium acetate as a buffer. It has been postulated that organic radicals are produced with simultaneous oxidation of the ferrocyanide ion to ferricyanide through a one-electron change (p. 60).
CHEMOTHERAPY
Potassium ferrocyanide has been suggested as a sedative and astringent for night sweats resulting from certain febrile diseases and as an antidote in iron and copper salt poisoning. It also is reported to have diuretic action. Certain cases of uticaria and pruritus have been treated successfully with sodium ferrocyanide.
As a 5 percent solution, sodium ferrocyanide has been administered intravenously in the study of glomerular function in normal children and adults and in cases of glomerulonephritis, hypertension, and tonsillitis. The injections were without toxic effect. The amount of the ferrocyanide excreted within the first thirty minutes (normally more than 25 percent) was independent of the volume of urine and served to indicate the extent of glomerular dysfunction. This test is said to be more sensitive than inulin clearance or creatine excretion, particularly for hypertension or tonsillitis.
Subcutaneous injections of sodium ferrocyanide every two days for two weeks desensitized rabbits after anaphylactic preparation. The salt also prevented direct shock in the rabbit from injection of foreign protein in the form of egg albumin.
Potassium ferrocyanide is reported to inhibit serum hemolysis, both in vitro and in vivo, and its administration to mice exerted a protective factor against a lethal dose of a hemolytic serum. Marked interference with the phagocytosis of bacteria was also reported. The effect of the salt is attributed to its charge relative to that of the reacting compounds.
CORROSION INHIBITION
Ferrocyanides and ferricyanides are said to exhibit interesting anticorrosion properties. Iron and steel articles that have been treated to develop protective coatings of iron phosphate may be treated further by immersion in a 5 percent ferrocyanide or ferricyanide solution, followed by drying and finishing as desired. It is said that these articles are then essentially noncorrodible.
The potassium salts are reported to be effective corrosion inhibitors in boiler waters. A recent patent states that a mixture of an alkali ferrocyanide, ferricyanide, or nitroprusside with a polyphosphate greatly reduces the corrosive action of water on condenser jackets, pipes, and other ferrous metal parts at temperatures above 180°F. Corrosion of mild steel, for example, due to inorganic salts contained in ordinary water, is quite serious at these temperatures. Polyphosphates alone are said to give only partial control. It is indicated that 5 to 50 percent of the complex cyanide on the weight of phosphate, or 0.05 to 0.5 grain per gallon of water is sufficient to achieve excellent corrosion inhibition.
DESULFURIZATION OF GAS
The patent literature describes two processes utilizing ferrocyanides for the removal of hydrogen sulfide from illuminating gas with the precipitation of elemental sulfur. In one process, an alkaline wash solution of potassium ferricyanide is used to absorb hydrogen sulfide and to oxidize it to free sulfur. The ferrocyanide, obtained during the oxidation, is then converted back to ferricyanide by electrolytic means.
In the other process, gases containing hydrogen sulfide are washed with a suspension of ferric ferrocyanide stabilized by ammonium salts. It is reported that sulfur is precipitated and ferrous ferrocyanide formed. The latter then may be oxidized back to the ferric salt by aeration of the suspension.
DETERGENTS
It is said that sodium ferrocyanide may be compounded with fatty acid soaps to produce a detergent mixture that does not lose its effectiveness in hard or sea water. The amount of ferrocyanide used depends upon the degree of hardness of the water, and its effectiveness is just as great if added to the water separately before addition of the soap.
DYEING OF TEXTILES
One of the first applications for prussiates, and still one of its widest uses, was in the dyeing of textiles. Complex iron ferrocyanides such as Prussian Blue may be fixed on the cloth by various mordants after solubilization with oxalic acid or ammonium tartrate. It also has been shown that the blue can be developed by passing the fabric into a solution of a ferric salt and then into an acidified solution of sodium or potassium ferrocyanide. With cotton and silk, ferric oxide is first precipitated on the fiber after which the passage of the fabric through the ferrocyanide bath results in the development of the Prussian Blue. Silks thus treated acquire additional weight as well as an excellent base for black dyes.
Fabrics may be dyed a lighter blue by working them in acidified sodium or potassium ferrocyanide solution, which is gradually raised to the boil. Oxidation of the ferrous ferrocyanide resulting from the decomposition of the prussiate has been accomplished by air or by other means. Similarly, acidified prussiates can be padded or printed onto cloth, which is then steamed to develop the color.
Sodium and potassium ferricyanide likewise have found application in dyeing, especially if reducing agents such as formic acid, hydroquinone, soluble starch, and sugars are used. A more recent process suggests the addition of acidified mercurous sulfate to the dye bath. Both in cold and boiling solutions a variation in the depth of the blue may be obtained by an adjustment in the concentration of the ferricyanide. Also, the substitution of ferrocyanides for ferricyanides is reported to produce lighter blues.
The chief role of prussiates in textile dyeing at this time is that of oxygen carrier in aniline black or certain vat dye formulations. Through the use of ferrocyanides these dyes are fixed more permanently on some types of spun rayon fabrics.
The “Prussiate Aniline Black” method of dyeing and printing is so named because of the sodium or potassium ferrocyanide contained in the formulations. The essential ingredients of these generally appear in the following ratio:
Aniline Hydrochloride (Aniline Salt)………………………………………..3 parts
Sodium Ferrocyanide (Yellow Prussiate of Soda)……………………….2 parts
Sodium Chlorate…………………………………………………………………….1 part
If the sodium ferrocyanide is replaced by potassium ferrocyanide, the print colors oxidize less rapidly, contributing to somewhat better processing. For stability, separate pastes are prepared, one containing the aniline salt, and the other a mixture of the chlorate and prussiate. These are mixed together just
prior to the printing, about 12 ounces of aniline salt being required per gallon of print color.
In many applications, the basic formulation is modified to improve the working properties and the quality of the black. The following is a typical recipe:
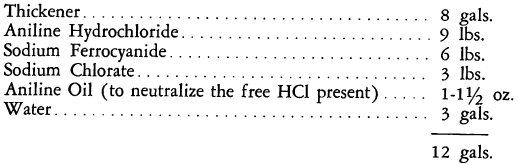
In nitroacetate aniline black formulations, the aniline salt is replaced by aniline dissolved in nitric and acetic acids. The proportion of ferrocyanide, however, remains substantially the same. The prussiate aniline blacks may be padded on cotton, linen, and viscose rayon goods in the usual manner.
In the case of some solubilized vat dyes that are insufficiently fixed upon certain spun viscose rayon fabrics, prints of good yield and fixation can be obtained by the use of the so-called “Ferrocyanide Process”. One suggested formulation of a dye paste contains 80 parts of ammonium chlorate (23° Twaddel) and 130 parts of sodium ferrocyanide (1:3) per 1000 parts of paste. After being printed, the dried goods are steamed for five to ten minutes in a rapid ager and are finally soaped at the boil in the usual manner.
For the discharge of indigo dyed or printed goods, a paste containing chlorate and a ferricyanide as oxygen-carrier is recommended, provided that colored discharges are not desired. Medium and light shades of indigo on cotton also may be discharged by printing of the goods with potassium ferricyanide followed by treatment with caustic soda. This process often is used in combination with azoic compounds of the β-naphthol class.
It is reported also that sodium ferrocyanide or ferricyanide is a good substitute for ammonium vanadate as used in some dyeing processes.
Soluble ferrocyanides and ferricyanides have been recommended for fixing certain colors such as aniline violet, magenta, and methylene blue on vegetable fibers. These same dyes also may be mordanted by the insoluble ferrocyanides of heavy metals, especially zinc.
ELECTRICAL EQUIPMENT
It has been reported that the zinc or cadmium electrodes of primary or secondary batteries, after amalgamation, may be protected further from attack during rest periods by a coating of paste which contains sodium or potassium ferrocyanide. It is said that this treatment also facilitates the charging of a secondary battery.
Potassium ferrocyanide has been used as an arc-stabilizing component in the coating of welding rods. As the proportion of ferrocyanide in the coating is increased, the nitrogen content of the deposited metal likewise becomes greater, while the carbon content is raised only slightly. Simultaneously, the terminal voltage necessary for the same quantity of welding current is reduced.
A recently patented process incorporates ferrocyanides to lower the electrical resistance of soils and of electrode-to-soil contacts. This use is suggested in the grounding of lightning rods and arrestors, transformer neutrals, radio and television receivers and transmitters, in the electrolytic protection of pipe lines from corrosion, and for ground return leads of various circuits. A gel of a heavy-metal ferrocyanide is formed in situ around the grounding rod by injecting or pouring either dilute or concentrated solutions of copper, nickel, or cobalt salts, and sodium or potassium ferrocyanide into position. The use of copper sulfate offers the advantage of economy. The two gel-forming components are employed preferably in gram-equivalent proportions, the solution of the copper salt being applied first. By the addition of 10-15 percent of ammonium hydroxide to the mixture of copper sulfate and sodium ferrocyanide, a single solution may be made, the gelation of which is delayed for periods of several hours to a few days. The lowered electrical resistance of the soil produced by such gels is reported to exist for periods of several years or more.
The oxidative effect of potassium ferricyanide in aqueous, alkaline solution is reported to be useful for reducing the diameter of fine tungsten wire by controlled dissolution of the wire. To avoid pitting and uncontrolled corrosion, it is necessary that the wire previously be worked to break down its crystalline structure.
ELECTROPLATING
The addition of ferrocyanides or ferricyanides of sodium or potassium to electroplating baths promises to effect substantial improvements in various plating processes.
These compounds, when used in tin electroplating baths, appear to sequester and remove iron and copper ions that are constantly carried into such baths and that probably act as oxidation catalysts for the stannous ion. Furthermore, even in the absence of copper and iron ions, the presence of the ferrocyanide or ferricyanide anions in stannous salt baths at a pH of 2.5 exerts a stabilizing influence and minimizes the formation of sludge. Although the ferrocyanide or ferricyanide addition has been found to be effective within rather wide limits (0.005 to 2 grams per liter), the preferred concentration is reported to be 0.01 to 0.5 gram per liter in a bath that contains a quantity of stannous compound equivalent to 15 to 45 grams of metallic tin. The following is an example of a dry formulation, prepared from anhydrous materials for dissolution in water to yield a bath having the desired pH:
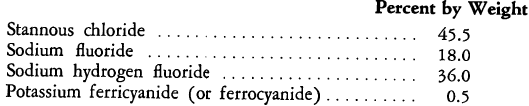
Such baths also may contain the usual organic addition agents and metal brighteners.
In silver plating, the addition of sodium or potassium ferrocyanide or ferricyanide to the strike bath for initiating the deposition of silver on base metal results in better plate adhesion and in improved bath stability. It also permits the employment of substantially more silver and less free cyanide ions than are now generally used in silver strike baths. In trials, the best results were obtained with a bath adjusted to a 20 to 30 percent plating efficiency, operated at a current density of 0.05-0.075 ampere per square inch of cathode area. A typical formulation contains 2-3.5 grams of silver, 45-50 grams of sodium cyanide, 60 grams of sodium carbonate, 15-50 grams of sodium or potassium ferrocyanide or ferricyanide per liter of bath solution.

MINERAL DRESSING
Sodium and potassium ferrocyanides and the corresponding ferricyanides are becoming increasingly important in the mineral dressing field. It has been found, for example, that the selectivity of flotation processes employing cationic reagents can be improved by inhibiting the ionization of the reagents. Potassium ferrocyanide, as a sequestering agent, has been suggested for this application.
Because of the polyvalence of soluble ferrocyanides and ferricyanides, these salts aid in the flotation of various sulfides and both unchanged and sulfided oxide, hydroxide, and carbonate ores. When introduced between the oiling operation and aeration, the soluble prussiates tend to improve froth flotation, increase the percentage extraction, and raise the rate of separation of the metalliferous particles. Although the formation of insoluble complexes can cause complications with some ores, those of copper may be processed effectively in alkaline solution.
Under certain conditions, the tendency to form insoluble complexes may be directed advantageously toward more efficient separation of mixed ores. For example, it has been found that the soluble prussiates tend to form relatively stable, insoluble polar films of copper ferrocyanide or ferricyanide on copper sulfide particles. As a result, flotation of these particles may be inhibited even in the presence of a usually effective collector. Accordingly, zinc blende and copper sulfide in ores can be separated differentially by depressing the copper sulfide and floating the zinc blende with nonxanthate reagents. Xanthates then may be used to destroy the polar film and thus permit flotation of the copper sulfide particles. In actual tests, an ore containing about 16.0 percent zinc, 13.8 percent copper, 7.1 percent iron, and 16.3 percent sulfur, was milled with sodium ferrocyanide and sodium carbonate to form a pulp with 30 percent solids. Differential flotation resulted in a zinc concentrate containing 75 percent of the total zinc and 6.7 percent of the total copper of the ore, and a copper concentrate with 83 percent of the copper and 13.1 percent of the zinc. It is reported that the sodium ferrocyanide alternatively may be added to the ore immediately before flotation or during the heating at sub-roasting temperature.
The benefication of low-grade nickel-cobalt ores occurring along with lead, copper, and iron, even when only 0.5-2.0 percent of cobalt and nickel sulfides are present, can be effected by flotation, using the ferrocyanides or ferricyanides of potassium and sodium. The copper-lead concentrate is floated by the usual reagents, e.g., dithiophosphate esters and commercial calcium cyanide. The tailings are treated with xanthate and an alcoholic frother after which the prussiates and an oxidizing agent are added to depress the iron. In this way the final flotation of cobalt-nickel concentrate results. In tests, the use of sodium ferrocyanide and potassium dichromate provided excellent recovery of the cobalt and nickel, while the further addition of potassium permanganate resulted in a concentrate having superior assay.
It is reported that when about 0.1 percent of potassium ferrocyanide is present in cyanide leaching solutions the dissolution of silver and gold is accelerated. The best results occur when the gold-silver ratio is greater than unity.
It has been found that ferricyanides also accelerate the rate of gold dissolution in cyanide leaching solutions. These complex salts may be prepared in situ by the addition to the cyanide bath of a ferrocyanide along with a suitable oxidizing agent such as potassium permanganate.
MIRROR MANUFACTURE
According to a recent patent, heavy-metal ferrocyanide gels, formed in situ, may be used to restrict to desired areas the deposition of reflecting coatings in the manufacture of glass and plastic mirrors. One side of a sheet of glass, for example, is treated first with a ferrocyanide solution and then with a cupric chloride solution to produce a stable, gelatinous coating. When the desired silvering solution is applied to the other side of the glass, the face of the mirror is fully protected. The insoluble ferrocyanide film then is easily removed by a water spray or by washing.
MORTAR
A rapid-setting, waterproof mortar has been described which may be prepared by mixing cement, cement and sand, or lime and sand with a potassium silicate solution to which is added potassium ferrocyanide or ferricyanide.
PESTICIDES
Diguanidine dialkali (sodium, potassium, or ammonium) ferrocyanides have been proposed as repellents for moth larvae. A recent patent reports the preparation of weather-resistant nicotine derivatives having important fungicidal and insecticidal properties. These may be made by the reaction of the nicotine salt of hydroferrocyanic or hydroferricyanic acid with a salt of iron, copper, zinc, or other heavy metal, mole for mole, at a pH of 5-7.5. Reaction of the solid heavy-metal salt with solid potassium or sodium ferrocyanide or ferricyanide and the respective nicotine salt also gives the desired product.
In the treatment of trees, vines, and shrubs infected with bacterial or fungus diseases, the injection of a mixture containing equal parts of ferrocyanide, ferricyanide, and potassium thiocyanate was proposed as a general stimulant to the plant tissues prior to the injection of specifics for the disease.
PETROLEUM
Ferrocyanides have been used in the petroleum industry for removal of trace metal ions such as copper, which are introduced into the oil for sweetening purposes. (p. 82).
Gasoline may be treated with aqueous potassium ferricyanide followed by a wash with sulfuric acid for sweetening and partial desulfurization. The effect of the ferricyanide is to oxidize free sulfur or sulfur dioxide that may be present.
PHOTOGRAPHY
The ferricyanide ion reacts with metallic silver to form insoluble silver ferrocyanide. Thus, potassium ferricyanide is widely used in photographic processes to bleach silver films and prints prior to further treatment. These then may be washed with solutions of various metallic cations to produce toning effects, or dipped into solutions of basic dyes which couple with the silver compounds to form brilliantly colored prints or films.
Soluble ferrocyanides are reported to be useful as fixatives in photography. The ferrocyanide ion apparently accelerates the reduction of the halides.
PIGMENTS
Prussiates for many years have held the prime position in the manufacture of Iron Blue pigments such as Prussian Blue, Turnbull’s Blue, and many others. The chemistry and art of making these pigments are very complex, and their discussion is beyond the scope of this book. Since many metallic cations form beautifully colored ferrocyanides and ferricyanides, however, the possibility of using these compounds as pigments is worth noting. For example, a complex potassium copper ferricyanide having a beautiful maroon color has recently been obtained. A description of most of the simple ferrocyanide salts appears on p. 38 ff.
PICKLING OF STEEL
Recent experiments indicate, and field reports confirm, the applicability of sodium ferrocyanide as a low-cost additive to steel pickling baths. In the pickling of steel, the use of sodium ferrocyanide (0.05-0.2 percent based on the total bath or 0.5-2.0 percent on the acid content) appears to exert inhibitory action but with no increase in pickling time. Microscopic examinations show that satisfactorily uniform surfaces result. Field reports state that after cold- working, these steels may have brighter surfaces. Furthermore, reduced consumption of acid has been reported.
The addition of up to 0.3 percent of sodium ferrocyanide to pickling baths generally regarded as “spent” but usually containing 4-5 percent of sulfuric acid, together with approximately 22 percent of ferrous sulfate, has been reported capable of extending the life of such baths. Laboratory tests with “spent” baths show that the time required for satisfactory pickling is reduced by 15-20 percent.
RUBBER
Potassium ferrocyanide acts as a peptizing agent in causing phase inversion of dispersions of water in rubber. Crude and reclaimed rubber may be blended and plasticized by milling, inorganic pigment being added if desired. In sequence, solid potassium hydroxide, a small amount of water, and oleic acid are worked in, which forms a dispersion of water in the rubber composition. Potassium ferrocyanide, as a 5 percent aqueous solution, is slowly blended into the mass, effecting an inversion of phase to form a rubber in water dispersion. Additional ferrocyanide solution and then more water are worked into the pasty mass to obtain the desired consistency. Dispersions prepared in this manner are reported to resist the “skinning over” that frequently occurs when latex emulsions are exposed to the atmosphere or when they are whipped to produce a foam rubber. It is probable that the peptization is effected by the polyvalent ion and that other soluble ferrocyanides would be equally useful in this application.
Potassium ferrocyanide and ferricyanide aid in the stabilization of both synthetic and natural latex foams permitting better control of gelation prior to the finishing operations. The final result is a sponge rubber essentially free from cracks.
Early patents suggest that the reaction product formed by the fusion of sodium ferrocyanide with sulfur may serve as a good accelerator for the vulcanization of caoutchouc.
Potassium ferricyanide and other water-soluble ferricyanides are effective as catalysts in promoting emulsion polymerization in various recipes for synthetic rubbers. These include butadiene-styrene, haloprene, acrylic, and other types of formulations.
SEPARATION AND IDENTIFICATION OF ORGANIC BASES
Many alkaloids form ferrocyanides or ferricyanides that are highly insoluble and that have characteristic crystal structures. A tabulation of the insoluble alkaloid ferrocyanides is found in Tables XI-XV (p. 51). The preparation of these salts and the examination of their crystal structures is a useful method for the detection of small quantities of alkaloids. Determination of the iron content of the salt can serve as an additional check.
Pyridine may be separated from homologous bases such as the lutidines, picolines, and collidines by taking advantage of the lower solubility of its ferrocyanide. A separation of pyridine and quinoline may be effected by precipitation of quinoline ferrocyanide from an acid solution of the bases by the addition of dilute ferrocyanide solution. After removal of the quinoline salt, additional ferrocyanide causes the precipitation of the pyridine salt. Quinoline and isoquinoline have been separated through precipitation of their mixed ferrocyanides by the addition of saturated sodium ferrocyanide solution to an aqueous solution of the mixed hydrochlorides containing an excess of the bases. On washing the crystals with water, however, the isoquinoline salt dissolves and the quinoline salt remains.
Pure diphenylamine has been isolated from a product contaminated with aniline by the addition of a saturated solution of hydroferrocyanic acid in alcohol to an alcoholic solution of the bases. The aniline salt is precipitated and removed, and the diphenylamine is recovered from the filtrate by treatment with caustic after concentration.
A method for the separation of o- and p-toluidines based on the selective precipitation of o-toluidine ferrocyanide has been reported. The mixture of bases is dissolved in hydrochloric acid until acid to Congo Red. A saturated solution of sodium ferrocyanide is added causing precipitation of the ortho isomer as the ferrocyanide. A similar procedure may be followed in the separation of a mixture of o- and p-aminoethylbenzenes. In this case, the salt of the para isomer is precipitated.
Benzidine is precipitated quantitatively from acid solution by sodium ferrocyanide. This can be adapted to a quantitative determination either of fer-rocyanide or of the base.151
TRACE-METAL REMOVAL
Both laboratory and field reports indicate that, under proper conditions, sodium, potassium, and calcium ferrocyanides precipitate traces of most heavy- metal ions from aqueous solutions, but are used commercially, primarily, for the removal of iron. These properties are particularly useful in purging fermentation media and in the purification of various industrial chemicals.
The yield of citric acid produced by fermentation processes, for example, is adversely affected by the presence of iron, zinc, manganese, and other heavy- metal ions in the cane molasses utilized. These cations may be removed from the fermentation medium by precipitation with potassium ferrocyanide so that substantially improved yields of citric acid are realized. The ferrocyanide is used to the extent of 0.4-1.2 g. per liter of molasses.
In the purification of another organic acid, a field report indicates that the addition of sodium ferrocyanide to the solution that is contaminated by between 400 to 500 p.p.m. of iron, removes iron down to 50 to 60 p.p.m. in one filtration. By evaporation and granulation, the iron content is finally brought down to 2 p.p.m. Any traces of heavy metals that are present are removed by the sodium ferrocyanide.
Another somewhat similar operation involves the addition of sodium ferrocyanide at two points in the process. In one filtration the iron content is reduced to 10 p.p.m. at a point where the concentration of the acid is approximately 14 percent. The solution is then concentrated to approximately 45 percent of its volume and treated further with sodium ferrocyanide, which again reduces the iron to 10 p.p.m. and removes whatever copper may have been picked up from the copper apparatus involved.
In the preparation of an organic vulcanization accelerator, treatment of the solution with sodium ferrocyanide equivalent to the heavy-metal ions present results in the production of a purer product.
In Tables VII and VIII (pp. 38 and 42) are described many salts of hydroferrocyanic acid which are so insoluble that the concentration of the metallic ion to be removed from the solution can be reduced almost to zero. Very few quantitative solubilities have been determined, but it is notable that the pH of the solution has a marked effect on the solubility of some metallic ferrocyanides.
Cadmium ferrocyanide, for example, is said to be soluble in concentrated hydrochloric acid but insoluble in dilute acetic acid. Lead ferrocyanide is very slightly soluble in water, but has a fair solubility in strong or weak acids. Stannous ferrocyanide has little solubility in dilute acid, but is slightly soluble in dilute ammonia. There are numerous other examples which indicate that the percent removal of cation from solution depends on the pH. Each system should be studied separately to determine the optimum conditions.
A number of specific applications have been developed for the utilization of sodium and potassium ferrocyanides in the removal of low concentrations of heavy-metal ions from solution. European wineries, for example, frequently use potassium ferrocyanide in beverage wines to remove sediment-forming heavy-metal ions and thus effect clarification. Some investigators reject this procedure, while a number approve but counsel caution by indicating limitations. Although hydrogen cyanide can be liberated in the mildly acid wine, it is reported that the amount would not be detectable unless a large excess of potassium ferrocyanide were used. Even then, it probably would be oxidized rapidly by air or transformed to the relatively harmless thiocyanic acid by traces of sulfur compounds. While in the United States such use of ferrocyanides has not been approved by the Pure Food and Drug Administration, a recently developed patented compound containing potassium ferrocyanide has been approved by the California State Department of Health for a year’s trial in California wineries.
It is reported that traces of copper remaining after the desulfurization of hydrocarbon oils can be removed satisfactorily by treatment of the oils with a 0.5-20 percent aqueous solution of sodium or potassium ferrocyanide at a pH of 1-5. Alternatively, since the traces of water needed to effect ionization are usually present in the oils, the latter may be percolated through beds of solid potassium or sodium ferrocyanide.
A German patent suggests the adsorption of hydroferrocyanic acid or its salts on a suitable porous carrier for use in freeing solutions from traces of iron ions.
What are ferrocyanides used for?
Synthetic asbolites for designs on ceramic ware have been compounded from ferrocyanides of cobalt, manganese, and nickel.
An easily separable radioactive iron, Fe59 or Fe55, was prepared in compound form when crystals or solutions of magnesium ferrocyanide were subjected to medium-intensity neutron bombardment.
Processes utilizing soluble ferrocyanides and ferricyanides have been proposed for developing a distinctive blue color on coal for trade-marking purposes or for masking an undesirable brownish cast.
After removal of iron from a mixture of yttrium and erbium salts, these rare-earth metals may be separated by fractional precipitation using potassium ferrocyanide.
