Table of Contents
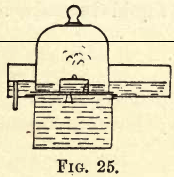
Nitrogen exists in the air, mixed principally with oxygen, so the simplest way to prepare it is to take away the oxygen from the air. Fill the pneumatic trough as before with water, and on the tray stand a bell jar, under which float a small porcelain dish containing a little amorphous phosphorus, as shown in fig. 25. Ignite the phosphorus with a red-hot wire; white fumes (P2O5) will be seen in the jar, but after a time will become dissolved in the water, and the water will gradually rise in the jar. When the combustion is over and the jar is cool, till two gas jars with water, place them inverted in the trough, and decant the gas from the bell jar into them, keeping their mouths under the water in the trough.
Nitrogen Gas Experiment I
Pass a lighted taper into one of the jars; the flame is extinguished and the gas does not take fire, showing that nitrogen is neither combustible nor a supporter of combustion.
Nitrogen Gas Laboratory Experiment II
Into the other jar pour a little clear lime-water, close its mouth with a greased cover-glass and shake briskly; the lime-water is not rendered turbid. This test distinguishes nitrogen from carbon dioxide.
