Gold crystallises in the cubic system, occurring frequently in nature in the form of cubes, octahedra and rhombic dodecahedra. Cleavage is never exhibited. Single detached crystals are comparatively rare, and the crystals are usually attached end to end, forming strings, and branching, arborescent, or moss-like masses, which are composed of microscopic crystals, usually octahedra. These forms occur frequently in quartz veins, but the single crystals, which are usually of larger size—viz., from ¼ to 1½ inches in diameter — are mainly found in drift deposits. They are rarely perfect or of brilliant lustre, although such crystals were found at the Princeton Gold Mine, Mariposa County, California, but occur more frequently with rounded angles, raised edges, and cavernous faces, which are often marked with parallel striations, and possess little or no lustre (Fig. 1). The octahedra found in California are usually flattened parallel to two opposite faces, or elongated, or otherwise distorted. Still more frequently they are only partially developed, as in Figs. 2 and 3. In all these cases “ the incomplete crystals have the appearance of a failure for lack of material” (W. P. Blake). Crystals of greater complexity, containing many modifying faces, occur chiefly in Siberia, Transylvania, and Brazil. The most common forms occurring naturally in Australia are the octahedron and the rhombic dodecahedron.
Liversedge found that, in the majority of cases, the gold embedded in massive quartz is remarkably free from any traces of crystalline form, and the larger the fragments of gold, the less crystalline form does it present. His observations enabled him to state that all well shaped crystals of gold appear to have been formed in what are now cavities, usually left by the removal of iron pyrites, or else in very soft matrices like iron oxides, clay, calcite, and serpentine. Crystallised gold is not usually met with in the quartz of the reef itself, but in the upper portions of the ferruginous and argillaceous casing of the reef and in the detritus near its outcrop.

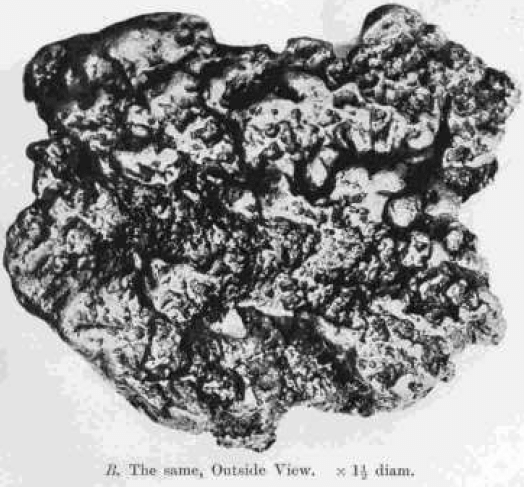
On the other hand, polished and etched sections of nuggets always show marked crystalline structure closely resembling that of a fused mass of gold. This is shown in Plate I. A, a section of a nugget from Coolgardie, Western Australia, which weighed 9.94 ozs., and consisted of gold 890, silver 105. Plate I. B shows the outside of the same nugget. There are no traces of the concentric structure which might have been expected if the nugget had been built up of successive coatings round a nucleus.

Artificial crystals can be obtained in several ways, but with some difficulty. The slow cooling of an ingot of gold from fusion usually gives faces and sometimes angles of octahedra on the surface of the metal. The presence of small quantities of copper reduces the size of the crystals. Feathery crystalline plates are precipitated in the electrolysis of a solution of chloride of gold and ammonium. By keeping an amalgam containing 5 per cent, of gold at a temperature of 80° for some days, and then digesting it at 30° with dilute nitric acid, bright crystals of gold can be obtained. In the Percy collection in the South Kensington Museum are some gold crystals found in the mercury troughs at the foot of the “ blanket strakes,” in an amalgamation mill. The troughs are placed so as to catch any stray particles of gold that may pass the blankets. As the amount of gold recovered in this way is very small, it is not worth while to clean out the troughs frequently, and in this case they had remained undisturbed for nine months, at the end of which time all amalgam was found to be crystallised. The mercury has been dissolved off by nitric acid, and the gold crystals remain. The smaller crystals are rather indefinite in shape, but amongst the larger ones (which are about half the size of a pea) are well-defined combinations of the octahedron, rhombic dodecahedron, and cube.
