Table of Contents
The extraction of silver by the solving processes simple. The ore is first roasted with salt in the usual way, whereby the formation of base metal chlorides cannot be avoided entirely. After roasting, the ore is first subjected to leaching with water, in order to extract the base metal chlorides, and then with hyposulphite of lime, to extract the silver.
Extraction of Silver
After a chloridizing roasting the ore should be examined to ascertain the amount of chloride of silver contained in it, according to 21. In case the extraction should not be satisfactory, it is then easier to find what the cause is. The ore is then prepared for leaching.
First Leaching
The roasted ore contains chloride of silver, which does not dissolve in water, but generally there are also base chlorides in it, as the chlorides of copper, zinc, lead, iron, antimony, etc., which are soluble. It is the purpose of the first leaching to extract these base metals by means of hot water. For this purpose the ore is introduced into a tub or square box of pine wood, the planks being one and one-half to two inches thick.

The boxes must be made as water-tight as possible and provided with a filter at the bottom. The filter is prepared in two ways, either as represented in Fig. 10 by fixing a false bottom, a, provided
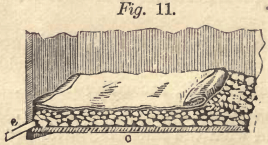
with numerous holes, one-half inch in diameter, about one inch above the bottom, b, or as Fig. 11 shows, without a false bottom. On the bottom, a, is thrown clean rock, quartz or poor ore of about the size of a hen’s egg, three or four inches high ; on this smaller stuff, and finally sand, free from mud. In Fig. 11, rock of about the same size is thrown directly on the bottom, c, spread four inches high, then a few buckets full of rock, not smaller than hazel nuts, and only so much of it taken as to equalize the surface. This is then covered with a piece of canvas and is ready for use. The boxes, according to their size and the weight of the ore, may contain from one to five tons. Generally the ore must not be over fourteen or sixteen inches deep, but some ore allows a good leaching with twenty-five inches.
The roasted ore, generally without sifting, is charged into tho boxes, and the surface spread evenly, leaving about six inches space from the top for the reception of the leaching water. The hotter the water is, the sooner it dissolves the soluble salts and the quicker the leaching progresses. It is conveyed through the pipe, d, and falls on a piece of canvas, whence it spreads equally and gently over the ore. The water soon reaches the bottom and begins to flow out through the pipe, e, into the trough, f.
In the beginning, the leaching water at e is highly charged with base metal salts, and shows a green color if there is much copper in the ore. The water is kept running in a continual stream till it reaches nearly to the rim of the box, when the influx and the efflux are equalized. After one or two hours a glass full of the liquid, at the pipe, e, is taken, and a few drops of sulphide of calcium (or of sodium) added. If a precipitate falls, of a dark or light color, the leaching must continue ; but it is not necessary to continue until no precipitate at all is perceived, as it requires some time— perhaps an hour—before all the water runs out after the pipe, d, is closed. The water which comes out last must be free from salts. This first leaching takes from two to four hours, sometimes longer.
Second Leaching
As soon as the ore is freed from the base chlorides soluble in water, a solution of hyposulphite of lime (70) is led in from a tub or tank, on the ore, in order to dissolve the chloride of silver. This leaching is conducted like the former. It depends on the amount of silver how long this work continues—from eight to twenty hours. The clear cold solution, containing the chloride of silver in the form of a double salt, has a very sweet taste, and is conveyed through a trough or indian rubber hose into a precipitating tub. Very rich ore, containing 12 to 15 per cent, of silver, would require forty-eight hours leaching, and even then it would be necessary to subject the ore to a second leaching with the hyposulphite, with an intermediate roasting with green vitriol and salt; for, with the best work, if 95 per cent, are extracted, the tailings would still appear sufficiently rich for this, containing about 200 ounces of silver per ton. Ores containing $350 per ton are often leached out perfectly in twelve hours. The end of the lixiviation is ascertained in the same way as in leaching with water, using the sulphide of calcium. If no precipitate is obtained the extraction is finished.
The color of the precipitate is a black-brown. The presence of base metals changes the color somewhat. Iron makes it black ; copper, red- brown ; lead and antimony, light red-brown, etc. The silver is first dissolved, especially if a diluted solution of hyposulphite of lime is used ; and for this reason the first precipitate is the richest in silver. Ore containing a great deal of lead— especially if the roasting was so conducted that a large part of it remained as sulphate of lead, which is not soluble in the leaching water—will give in the beginning of the leaching with the solvent a precipitate of silver with some lead ; afterwards, however, the silver diminishes, so that the precipitate of lead finally appears free of silver. Besides the sulphate of lead, sub-chlorides and oxy-chlorides are formed during the roasting which are not soluble in water, but are dissolved by the hyposulphite of lime ; for this reason always some base metals will be found in the precipitate.
In case rebellious ores are treated, and hot water is used for the extraction of base chlorides, a better silver is obtained if the ore is cooled down by cold water before the cold and diluted solvent is applied. Purer ores may be treated with a warm solution of the solvent.
When examining the tailings as to the amount of silver left therein, it must be remembered that; after leaching out a quantity of metals by water and the solvent, the ore lost a considerable part of its original weight, and that consequently one-half ounce of such tailings taken into assay will always give a larger silver button than there ought to be. A true assay of leached tailings is made if half an ounce of the same ore is leached on a filter with hot water and hyposulphite of lime, in the same way as the ore on a large scale, washed with water, dried and weighed. The weight found after leaching must be taken for half an ounce in assaying the tailings.
The residue, or tailings in the leaching box, must be removed now as valueless. The sides of the leaching boxes are from eighteen to twenty-four inches above the bottom, and being from six to eight feet square in the clear, the removing of the tailings by means of chloride is easily effected. The men must be careful not to dig too deep, otherwise the filter will be injured. It is quite proper to fix wooden staves, as long as the box requires, on top of the filter. These staves are one inch wide and one-half of an inch thick, and are placed from four to five inches apart, so as to protect the canvas or filter against the shovel. In Fig. 11 the staves are laid upon the canvas.
The leaching boxes or tubs may be arranged so that, being tipped over, the whole charge falls out at once. In this case the filter must be made in a different way from that described above. Rocks are not serviceable here. On the false bottom, a, of Fig. 10, a layer of thin, leafless switches is placed, and on this another one crosswise, then covered with a piece of canvas, and secured with some staves to prevent the falling out of the whole filter when turned over.
Precipitation of the Silver
The liquid of the second leaching is conveyed through a trough or india rubber hose to the 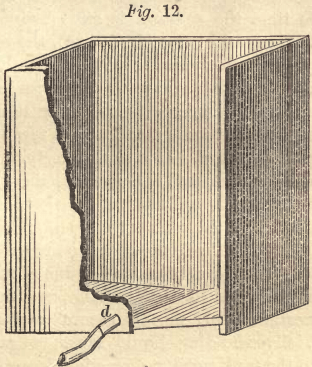 precipitating tanks, of which three or four are employed. If tubs are used, which for this purpose are the best, they are from three to four feet in diameter, and four feet high. The tanks or boxes have a rectangular shape of about the same capacity, the bottom being inclined toward the middle, as shown by Fig. 12. The hyposulphite of lime, as it comes from the leaching tanks, is conducted into these until they are more than two-thirds full. The trough or hose is then changed to discharge the liquid into the next precipitating tub, while the precipitation of the first commences.
precipitating tanks, of which three or four are employed. If tubs are used, which for this purpose are the best, they are from three to four feet in diameter, and four feet high. The tanks or boxes have a rectangular shape of about the same capacity, the bottom being inclined toward the middle, as shown by Fig. 12. The hyposulphite of lime, as it comes from the leaching tanks, is conducted into these until they are more than two-thirds full. The trough or hose is then changed to discharge the liquid into the next precipitating tub, while the precipitation of the first commences.
The liquid used for precipitating the silver is sulphide of calcium (69). It is poured in until all the silver is supposed to be precipitated, and at the same time the solution is stirred vigorously. Treating always the same kind of ore, the required quantity of the precipitating agent is soon learned. The black precipitate sinks to the bottom, and the workman now dips a little of the clear liquid out in a glass tube or tumbler, and adds a few drops of the sulphide of lime. If a dark precipitate or a dark color is produced, it shows that there is still silver in the liquid, and more of the agent must be added ; but if on the contrary no precipitate is observed, there is either enough or too much of the sulphide. To prove this, some of the silver-holding liquid is added to a test, taken from the tank under treatment. If in this case a precipitate is formed, silver-holding liquid must be carefully added to the tank until no reaction is produced. This work, delicate as it seems, is easily learned by the workmen. If a little silver should be left in the liquid, it is not injurious, neither is the silver to be considered as lost, because the same liquid is used over again ; but a small excess of the sulphide of calcium would cause a loss in silver, as it precipitates sulphide of silver in the leaching tank in the mass of ore, which is not dissolved again. The precipitation is performed in a short time, requiring about fifteen minutes for each tank. The stirring must be executed with vigor. Wooden grates fixed to a vertical stem will answer the purpose.
The clear solution above the settled precipitate is pumped or elevated to the reservoir, whence it was conveyed on the ore. It is now ready to be used again. The sulphide of calcium having performed its duty in precipitating the silver, is turned into hyposulphite of lime, thus replacing all of the solvent.
To prevent small floating particles of silver from being elevated with the liquid, it is well to allow sufficient time for the precipitated silver to settle. For this reason it is better to have more precipitating tanks or tubs. It is not necessary to remove the silver after each precipitation. The clear liquid can be drawn off, by means of a syphon, from all the precipitating tubs into a general receiver, whence it may be pumped up. After the solvent has been removed, the precipitated silver can be drawn off through the pipe, d, Fig. 12, directly into canvas bags.
Treatment of the Precipitated Silver
The black precipitate of sulphide of silver is conveyed directly into filters made of canvas, either in the shape of pointed bags, like those used for amalgam, or in the shape of common bags. As soon as all the liquid runs out, pure water (if possible, warm) is poured on the silver, and this repeated several times till no taste is observed in the filtering water. The precipitate, while still in the bags, is placed beneath a screw press and the fluid pressed out as completely as possible. The black silver cakes are then taken out and dried in a warm room or in a drying oven. For the purpose of burning off the sulphur, the dried sulphide is introduced into a muffle or other calcining furnace, and heated till the sulphur commences to burn with its known blue flame. When this disappears the heating must continue at a dark red heat for one or two hours. By this operation the cakes are reduced almost entirely to metallic silver, generally covered with threads of silver ; sometimes an intense green color is assumed by pieces remaining in the furnaces over night.
The burned cakes are now prepared for smelting in crucibles. They are placed in black lead crucibles, according to the size, up to three hundred pounds, and fused. All the sulphur was not driven out by the preceding operation. The remaining part must be removed by placing metallic iron (5, d) in the fused metal; thereby iron matt is formed, which rises to the surface and is skimmed off. The surface of the silver is then cleaned by adding some bone ash and borax, or borax alone, which is also skimmed off and the silver dipped out or poured out into moulds. According to the careful treatment in the roasting process, and the nature of the ore, the silver will be from 800 to 950 fine.
Mr. O. Hofmann, in need of sulphur for the production of sulphide of calcium, used to calcine the dried sulphide of silver in iron retorts. In this way he obtained a large proportion of sulphur as a fine sublimate. This could be done also in a proper muffle furnace, so arranged that after all obtainable sulphur had sublimated in a receiver this could be removed and the calcination continued under access of air.
https://www.911metallurgist.com/precipitation-copper-contained-silver-leached-copper
Quality of Ores fit for the Solving Process
There is no process so suitable for all kinds of ores as the solving process. Generally considered, all silver ores can be treated by the solving process which are subjected to the pan amalgamation after roasting ; 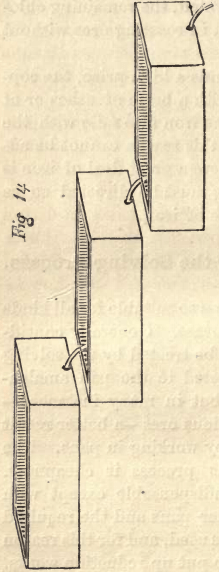 but in many instances— especially with the rebellious ores— a better result is obtained by this than by working in pans. The great advantage of this process is cheapness. Roasting of course is indispensable except with chloride ores ; but neither pans and the required power, nor quicksilver, are used, and for this reason less capital is necessary to put up reduction works. All the cupreous silver ores of Cerro Gordo, Yellow Pine, Montgomery, and of the other now silver districts, can be treated to great advantage by the solving process.
but in many instances— especially with the rebellious ores— a better result is obtained by this than by working in pans. The great advantage of this process is cheapness. Roasting of course is indispensable except with chloride ores ; but neither pans and the required power, nor quicksilver, are used, and for this reason less capital is necessary to put up reduction works. All the cupreous silver ores of Cerro Gordo, Yellow Pine, Montgomery, and of the other now silver districts, can be treated to great advantage by the solving process.
Two objections have to be considered. First, there is more water required than for pan amalgamation—at least this is the case with rebellious ores ; but the quantity of water depends on the quantity of base metals in the ore, and also on the arrangement of the leaching boxes. One box, containing one ton of ore, requiring three hours leaching, may consume 250 gallons of water; three boxes of the same size would take three times as much water if placed on the same level; but by arranging the boxes in a less favorable position, one above the other, as shown in Fig. 14, only one-half of the quantity of water is needed. It takes more time to leach three boxes together than a single one. Leaching the ore with hyposulphite of lime, the supply of this in the first box must be stopped if no more silver comes out, and the solution carried, by means of hose, to the second, and then to the third box, if it should be necessary; so that while the second box is yet under leaching, the first can be discharged and a new charge introduced. All three boxes should receive clean water at the start. This arrangement should be adopted only when rendered necessary by the scarcity of water. To have all leaching boxes on a level is preferable.
The other objection is confined to a certain class of ores containing clay and lime. If pulverized, so much fine pulp will be produced that the leaching is impossible. It is not advisable to crush the ore coarser than will allow of its passing through a sieve of forty holes to the inch, in some cases, perhaps, through thirty-five holes; and if with such crushing a fine clay pulp is produced, the ore is unfit for all leaching processes, unless wet crushing is adopted, in order to separate the slime from the sand, as Mr. O. Hofmann was compelled to arrange in Trinidad, Sonora. In this case, a separate drying for the purpose of roasting is not necessary if long furnaces are in use. It is not unlikely that for similar ore and pan tailings an agitating filtering box could be constructed which would render the leaching possible.
Sulphide of Calcium
Sulphide of calcium for the precipitation of silver is preferable to the sulphide of sodium, principally for the reason that its manufacture is cheaper and more easy, but also on account of the quality of the precipitated silver, which is easier to wash, to press and to desulphurize. The sulphide of calcium is easily obtained and manufactured on the ground where the mill is situated. The articles required for this purpose are brimstone (worth about four cents per pound) and burned lime. The sulphide is formed only from caustic lime, consequently more is obtained from fresh burned lime. Of this a certain quantity is charged into an iron kettle, water added, and then the pulverized sulphur. The proportion of sulphur and lime depends on the quality of the latter. The purest quality of lime from Santa Cruz, Cal., for instance, takes one pound of sulphur to 1.33 of lime. Of poorer qualities of lime it is better to take three pounds to one of sulphur and about ten parts of water. It is kept boiling for two or three hours, stirred with wooden shovels, and then discharged into a filtering box, prepared like Fig. 10, 61. The clear, dark, yellow-red sulphide of calcium comes out from below the filter, and can be kept in iron vessels. The liquid ought to be between 5° and 6° Beaume. The residue is washed with water, whereby a diluted fluid is obtained, which is used with the lime of the next charge. Mr. E. Smyth, in La Dura, Mexico, treats the lime and sulphur with steam. This has the advantage of dispensing with the stirring, and may be performed also in wooden vessels. The steam replaces the fire and has no chemical influence on the quality of the sul-
