Table of Contents
Changes in coke hardness affect the working of the blast furnace, for soft coke is an obstacle to proper furnace operation. Soft coke is due to a low hydrogen-oxygen ratio in the coal charged; increasing this ratio increases the hardness of the coke. The best means of determining the hardness of coke is the combined tumbler and ball-mill.
WHAT IS GOOD COKE
It is not always appreciated that the correct answer may be such a reliable forecast to the physical condition of the blast furnace, and incidentally its entire operation, that among the ordinary variations in other raw materials and also in operating conditions, it assumes a position of primary significance. Inasmuch as coke forms two-thirds of the total bulk of material charged, this assertion will not seem exaggerated, and in the subsequent discussion its applicability will be presented.
Coke is the ideal blast-furnace fuel because it is strong and hard enough to resist the crushing and grinding effects of the furnace burden, and its porosity allows the ready passage of gas upward through the charge, and rapid combustion before the tuyeres. Coke deprived of its strength and with a cell structure physically frail and chemically susceptible to solution by carbon dioxide has its chief asset eliminated. When the melting zones are approached the fluid cinder trickling toward the hearth will envelope the small pieces of coke and form a sticky mass that will retard the free passage of gas. Blast pressure will then rise, driving will slow up and fragments of coke will lie inert before the tuyeres, rendered practically incombustible by their slaggy envelop. It is believed also that coke messes at cast are attributable to quantities of such coke floating unburned in the hearth.
It has been correctly claimed that coke rendered small outside the oven possesses greater combustibility than that so developed in the coking process. While this is true of reduction in size by ordinary breakage or shattering, where the porous inner cell structure is cleanly exposed, it does not hold true of coke that is ground small, for the grinding action causes the fine dust-like particles to enter the cells and so form a dense and quite incombustible surface.
SATISFACTORY TEST FOR HARDNESS OF COKE
The importance of initial size and the resistance of coke to reduction in size has been recognized, but methods thus far employed to determine the physical nature of coke have not given full satisfaction. The shatter test, now largely employed, usually consists in dropping 2-in. coke four times upon an iron plate from a 6-ft. (1.8 m.) elevation and then screening through certain sizes of mesh, the various sizes thus separated being reported as percentages. This gives an indication of the condition of the coke when it reaches the furnace, having passed through the four major stages of handling; viz., cars to bins, bins to larry, larry to skip, and skip to bells.
But it must not be supposed that reduction in size ceases when the bells are lowered and the coke passes to the furnaces. It will continue and may even be accelerated throughout the entire passage from stock line to tuyeres. The gases will endeavor to disintegrate the cell structure and the mass of stock will exert a destructive grinding and crushing effect. Therefore, while a shatter test may indicate the extent of size reduction due to handling, it does not show the condition of the coke when it reaches its field of action—the tuyeres. Indeed there is frequently misguidance when the shatter test is depended on to determine fit or unfit—hard or soft—blast-furnace coke.
If a shatter test shows high fragmentation, the coke may have a weak, chalky structure that will resist no physical strain and cell walls susceptible to carbon-dioxide solution; or the coke may be glass-like in its brittleness but strong enough to bear the grinding, crushing, and abrasion of the brickwork, limestone, scrap iron, etc., on the way to the tuyeres, maintaining throughout a size that permits it to function properly as a porosity medium and that precludes the likelihood of its being “drowned” in slag. The coke that resists shattering is either the tough, soft variety, which is sure to yield to the abrasive action later on; or it is the strong, hard kind that is not susceptible to any sort of physical attack. The need for a more reliable test is, therefore, quite evident.
The effort to apply a test that more closely follows the treatment of coke in the furnace was stimulated by the article of G. D. Cochrane in which he compares the results of a tumbler test on coke with the operation of a low-pressure furnace. In following his lead, we have used a combination tumbler and ball-mill to determine the hardness of daily coke to three 500-ton furnaces, D, E, and F. The bosh angles of the furnaces are 76°, 76°, and 73½°, respectively.
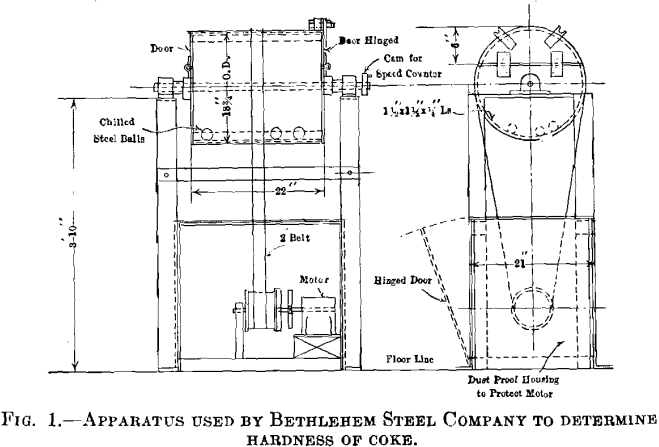
Our apparatus, shown in Fig. 1, is a 22-in. (56 cm.) steel drum, 18¾ in. in diameter with doors at either end and four 1½-in. angles riveted inside longitudinally. A ¼-hp. motor drives the drums at about 20 r.p.m., the belt passing from the motor gear train over the drum itself. Thirty pounds of dry 1-in. coke about half fills the drum. This sample is tumbled for 1250 revolutions (at 20 r.p.m.) with eleven 1¼-in. (32 mm.) steel balls. The resulting fines are screened through ½-in. mesh, and the remaining portion is weighed and reported as per cent, of original. This quantity is termed the coke hardness number.
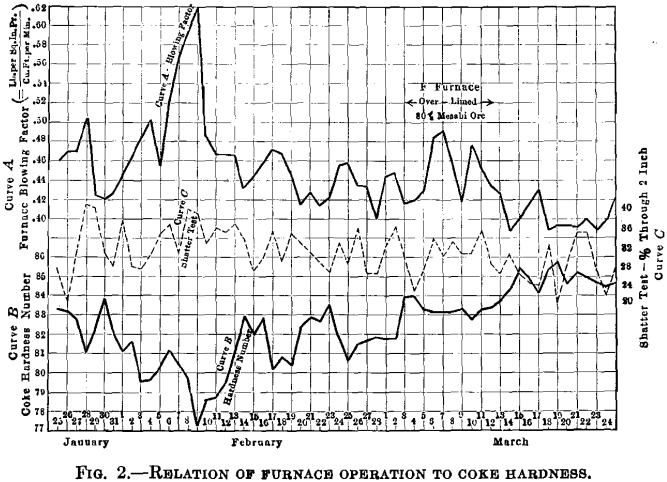
The results of the regular shatter test are shown in Fig. 2 as per cent, through 2-in. mesh, and after four 6-ft. drops upon an iron plate. The results of both the tumbler test and the regular shatter test were compared with furnace operation on each subsequent day when the coke tested was passing through the furnace. A period of two months was selected as typical of conditions at that time, being marked by irregularity both of raw materials and in furnace behavior. The hard ores, for example, varied in furnace D from 0 to 100 per cent.; in furnace E, from 0 to 20 per cent.; and in furnace F were fairly constant at 25 per cent. Tons of stone used per ton pig produced varied widely, the daily averages covering a range of 0.40 to 0.65 ton. The daily averages of scrap charged per ton pig produced ran from 0.08 to 0.35. Coke consumed per ton pig varied as much as 20 per cent, on each furnace. The operators fought the wide variations in blast pressures that were constantly threatening, and maintained an average pressure of 17 lb. (7.7 kg.) for the period.
The blowing factor is simply the ratio between the blast pressure and blast volume:
Blowing factor = average blast pressure, lb. per sq. in./average wind blown, cu. ft. per min. x 1000.
In no way is the physical well being of a furnace so truthfully indicated as by the blowing conditions. The blast is the pulse of the furnace. Pressure and volume conditions must be considered simultaneously, however, for the furnace may be driving well with pressures up to 18 or 20 lb. due merely to rapid blowing; or it may be hanging and slipping and may require slackening of the engines with but 14 or 15 lb. pressure. High pressure and low volume produce a high blowing factor and indicate poor working; low pressure and large volume yield a low blowing factor and show proper furnace conditions. As a matter of daily record, blowing conditions furnish more reliable data on the health of the furnace than either tonnage or rounds charged, both of which depend largely on the nature of the ores used or the size of burden; and both are affected by delays. Moreover, both tonnage and rounds charged, other conditions being constant, are direct consequences of blowing conditions.
The almost unfailing inverse coordination, day by day, between the hardness number and the blowing factor was enlightening, particularly in the face of the numerous operating irregularities that marked the test period. It was only between Mar. 5 and 10, when furnace F was carrying 80 per cent, of a Mesabi ore that has always caused trouble, that the furnace worked badly in spite of fairly hard coke. Hardness number was between 83 and 84 at the time. Cold stock reaching the hearth, due to heavy slips, led to off casts and large excess of stone was carried to curb the rising sulfur in the pig; this further aggravated high pressure conditions.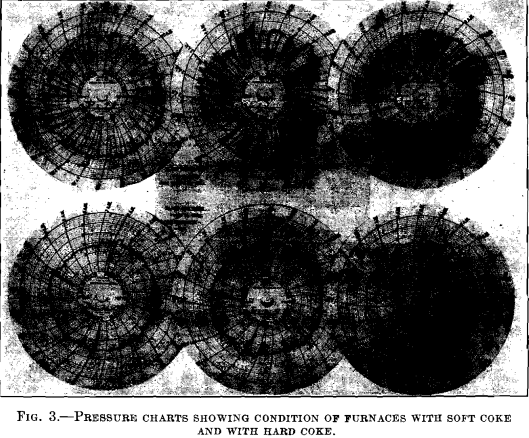
The two sets of pressure charts shown in Fig. 3 give evidence of the condition of the furnaces when working with the soft coke and with good, hard coke. The conditions on Feb. 7 and Mar. 21, as shown in Fig. 2, should also be compared.
It would be absurd to claim that hard coke alone is the remedy for all blast-furnace maladies, for furnace operation fluctuated when the coke hardness did not. But there was no material change in coke hardness that did not show its effect upon the furnaces. The curves in Fig. 2 are comparable day for day, the coke data having been advanced by one day.
What has been said concerning the shatter test must not be construed as condemnatory of its general value. Fig. 4, showing the direct relation to furnace blowing factor of the shatter test and hardness number, respectively, demonstrates the coordination between furnace conditions and the quality of coke. That the furnace is more sensitive to change in the hardness of coke (tumbler test) than to change in the toughness of coke (shatter test) is shown by the daily comparison between hardness and furnace condition; see Fig. 2. For example, on Jan. 28, the shatter tests indicated the most brittle coke of the entire test period, 40 per cent, through 2-in. mesh, but the furnace operation was normal, as registered by a blowing factor of only 0.480. About Feb. 8, the shatter test again showed 40 per cent, through 2-in. mesh, but this time the furnaces were in poor condition; pressures were up to 25 lb. and the blowing factor reached 0.620. The best furnace week was that of Mar. 21, when the blowing factor was steady at 0.400 for seven days, but the shatter test showed both unwarranted variation and magnitude, being as high as 35 per cent, through 2-in. In each instance the hardness number was in accord with the furnace operation.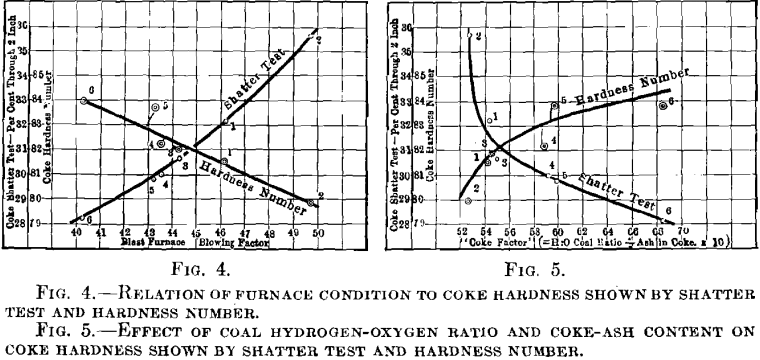
Hard coke means satisfactory furnace operation. Below a hardness number of 81, the furnace showed very seriously the effect of soft coke. Hardness over 85 does not show any particular effect, good or otherwise, upon furnace conditions. Beyond a doubt what must be sought in the furnacing quality of coke is its resistance not only to fragmentation but to abrasion. This characteristic is well indicated by the tumbler test. The use of steel balls in the tumbler is advantageous, as in the furnace the coke is in contact with masses of greater density than itself. The introduction of hot carbon-dioxide gas would add both interest and complexity to further investigations.
The numerical value of the blowing factor is affected by the initial size of the coke. If more than 45 per cent, passes through a 2-in. mesh, it requires little further reduction in size to tie up the furnace with a bad case of “small-coke indigestion.” Hence if numerical comparison is sought between furnace behavior and the hardness of a coke that varies greatly in initial size, that item must be taken into consideration. During the test period, there was no marked variation in size.
CONDITIONS AFFECTING HARDNESS OF COKE
While the coal used is the most important factor in determining coke quality, it is difficult to establish a rule governing the quality of coke resulting from different coals, particularly those recognized as coking coals. We have verified the theory of David White that the hydrogen to oxygen ratio on a dry-coal basis affects the resulting coke quality, even substituting for quality the unpretentious term hardness, and though limited to but 20 points variation in the ratio (72 to 92 per cent.) as compared with his range of 10 to 290 per cent. Average values for successive 10-day periods show rather steady increase in both hydrogen-oxygen ratio for the coal used, and the hardness of the coke produced.
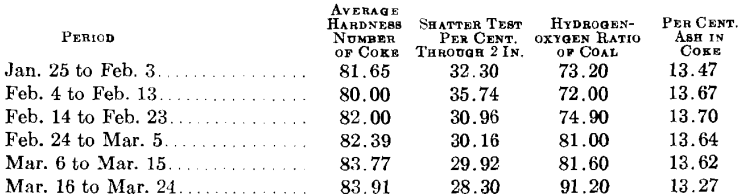
Doctor Cochrane has shown the detrimental effect of ash in coke upon its hardness. The variation of ash in our coke, though small, was sufficient to corroborate Cochrane’s claim, and ash content has been considered in this investigation as second among conditions that influence quality.
The third and final consideration is that of oven practice, as represented by the heat applied in coking. As heat is maintained in the empty ovens during slack times and as 75 per cent, of the coke was pushed on day shift, with an average coking time of 33 hr., some of the coke was short-period coke, and some long-period coke. The reported heats, in British thermal units per pound coal charged, are not deemed sufficiently accurate for purposes of direct comparison throughout the test period. We are satisfied, however, that overcoking has a detrimental effect on hardness; that while overcoking increases the depth of dense surface, it weakens the cell walls and structure, rendering the coke susceptible to attrition. The unprecedented drop in coke hardness from Feb. 5 to 10 is attributable largely to overcoking, there being an increase from about 1250 B.t.u. per pound coal during the previous 10 days, up to about 1500 B.t.u. during the period Feb. 5 to 10. The coal used during this period exhibits no great variation over that charged immediately previous or subsequent thereto. The hydrogen-oxygen ratio was low (72 per cent.) but nearly constant. During the final 10-day period of the test, the number of British thermal units per pound coal again rose to about 1500, and served to counteract the effect of the high hydrogen-oxygen ratio, at this time about 90 per cent., in raising the coke hardness. No data are as yet available on the hardness of green coke, but it is reasonable to believe that such coke will be soft. In undercoking, strong cells walls have not yet been established; in overcoking, the cells have been enfeebled. Comparisons have been drawn between the hydrogen-oxygen ratio in coal and ash in coke, on one hand, and the hardness of coke on the other, averages being taken over successive 10-day periods. The hydrogen-oxygen ratio and ash have been incorporated into a factor:
hydrogen-oxygen ratio/per cent. ash in coke x 10 = coke factor
which is plotted against both tumbler and shatter tests, in Fig. 5.
With fairly uniform coke ash, as the hydrogen-oxygen ratio drops below 74 per cent., the hardness of the resulting coke falls off rapidly; as the ratio exceeds 87 per cent., the effect on coke hardness becomes less and less marked.
That stocking coal affects the hydrogen-oxygen ratio is shown by the following: A 9-mo. stock of Davis coal showed a ratio of 102 per cent., while Davis coal direct from the mine showed a ratio of 125.8 per cent. Some 9-mo. stock of Fulton coal showed a ratio of 55.1 per cent, while Fulton coal direct from the mine showed a ratio of 57.5 per cent. It was the steady increase in the use of fresh coal that brought about the regular rise of the hydrogen-oxygen ratio throughout the test period.
SUMMARY
Coke hardness has an extremely marked effect upon blast-furnace health. The furnace is more immediately sensitive to hardness, which resists wear and tear within the furnace, as shown by the tumbler test, than to toughness, which resists rough handling without the furnace, as shown by the shatter test. The tumbler test is therefore superior for daily control; its adoption is also recommended on grounds of simplicity, reduction of the personal equation, and the saving of labor.
The shatter test is of value as a matter of general record but does not surpass the tumbler test in this respect.
Both tests for coke hardness show reliable coordination with the hydrogen-oxygen ratio of the coal used; the higher this ratio, the harder the coke.
Blast Furnace Coke
While determining the furnaceability of coke it is important to bear in mind the fact that combustibility plays a considerable part in blast-furnace working.
This test is a step in the right direction and will show good comparative results for a given coke at a given furnace; but a person must be careful about saying that the harder the coke the better for any furnace plant. Coke made from 80 per cent. Pittsburgh coal and 20 per cent. Somerset coal is a very hard strong coke, but it is quite dense and in large pieces. In a blast furnace, like some eastern Pennsylvania furnaces, making about 200 tons per day, this coke did not give good operation. The blast pressure dropped, the furnace was hot on top, and it made high-sulfur iron. With coke from 100 per cent. Pittsburgh coal, the same furnace made good low-sulfur iron and the top was cool. That straight high-volatile-coal coke would have shown less hardness in the tumbler barrel than the 80-20 mixture; but for that furnace it was better because it was more combustible.
The coke is rather friable at Bethlehem; the stronger they get it the nearer it comes to being right for their big furnaces. At a plant like the Jones & Laughlin Co., in Pittsburgh, the same thing might be true; a little stronger coke would be better for their furnaces. On the other hand, for the smaller furnaces or where the coke conditions are such that the coke tends to be stronger and harder anyhow, there is a possibility of getting it too strong. So the test should be judged on the basis of the coals used and the furnaces using them. How was the coke sample used for the test taken? Through what mesh was the 30-lb. sample screened?
The coke sample is always taken from the car. A bucket about 1 ft. deep and 1 ft. in diameter is clamped on the rail below the hopper; then when the car is opened, a lid on the bucket is slowly drawn open with a rope. The operator stands some distance away. He tries to get a representative sample through the depth of the car.
The 30-lb. sample was made up of material that passed through a 2-in. mesh and remained on 1-in. mesh.
Blast-furnace managers speak in general of the combustibility of the fuel as merely a qualitative term; we need quantitative data by which we can say that coke made at a certain temperature has a certain solubility in carbonic acid, so that we know exactly how quickly it acts upon the carbonic acid to reduce it in the furnace. The term “solubility of coke in the upper part of the furnace” has always struck me as being exceedingly indefinite and unsatisfactory and should be made precise.
The testing of coke for its ability to carry a burden can be somewhat simpler for foundry purposes than in the case of the blast furnace, as the column to be supported is seldom over 25 ft. high. The reason for such a short height, as compared with the great heights in a blast furnace, is that within the 15 to 25 ft. charged in a cupola there is enough metal to absorb sufficient heat from the waste gases to allow the escape of only sufficient heat up the stack to give the proper draft. The test described for coke is a very good one not only for blast-furnace purposes but also for foundry coke. I would recommend, however, that the quantities used be larger, necessitating a tumbling barrel of larger capacity.
Is a 30-lb. sample adequate for all classes of work? Some put the standard as high as 1500 pounds.
The larger the sample is the better; we use a 50-lb. sample for the shatter test and about 250 lb. for the screen test. We take a bucket full from each car and the moisture sample is run on a higher amount. The coke is placed in pans about 2 ft. long and 8 in. wide. Then one pan out of every three is selected for the screen test.
