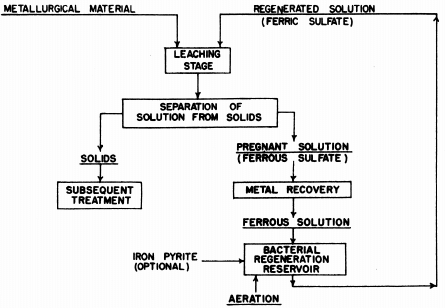
USING BACTERIA to leach and recover a metal from any metallurgical raw material with a ferric sulphate sulphuric acid lixiviant has been patented by Kennecott.
Kennecott Copper Corp. just recently obtained a U.S. patent on bacteria leaching of ores containing sulphides and/or iron sulphate. According to Stuart Zimmerley, director of research and one of the inventors, Kennecott found that several strains of iron-oxidizing autotropic bacteria occur naturally in mine waters at Bingham Canyon and Chino.
These bacteria are able to live and multiply in relatively high concentrations of dissolved copper, and can be used to leach and recover copper, molybdenum and zinc from their sulphide or mixed oxide-sulphide ores. In addition, processes have been developed that use bacteria to upgrade iron-chromite and iron-titanium materials by leaching out the iron.
How the Process Came About
Kennecott’s processes involves the use and continual regeneration of a ferric sulphate sulphuric acid lixiviant which has been inoculated with iron- oxidizing autotropic bacteria tolerant to relatively high concentrations of the particular metal or metals being sought.
For many years, waste water flowing from certain copper mines has been deliberately percolated and re-percolated through waste ore dumps and even back through the worked out areas of the mine to leach out whatever copper values might be contained. Copper has been recovered by scrap iron precipitation. In some cases very low grade ore deposits have been leached in place in this manner, but, generally speaking, this procedure has been regarded as far too slow to be economic if any other method is applicable at the same time.
Ferric sulphate and sulphuric acid naturally present in the mine water is responsible for the solution of the copper. Ferric sulphate is also an effective lixiviant for a number of other metals. For example, metallic copper and iron are readily attacked by a ferric sulphate solution. Recovery of metal values from certain secondary or scrap materials, and upgrading of metallurgical products after a reducing roast are possible through ferric sulphate leaching.
Iron-bearing chromite and titanium materials can be roast-reduced and the metallic iron removed by ferric sulphate leaching. While lead sulphate is not readily soluble in brine solution alone, it is if ferric sulphate is present in the brine. Ferric ion in solution has proven to be valuable in the purification of electrolytic zinc and manganese solutions—the ferric ion serving as a carrier, removing the impurities from the solution.
In all these uses, the ferric ion is changed to ferrous by reason of the metallurgical reactions taking place during the leaching procedure.
Principal deterrent to any widespread use of ferric sulphate-sulphuric acid leaching, has been the need to regenerate the spent lixiviant before recycling it to fresh feed. In acid solutions, regeneration poses a considerable problem. Atmospheric oxidation of acid ferrous solutions to the ferric state is slow and not aided materially by aeration. While regeneration has been accomplished by the use of SO2 and aeration, use of ferric sulphate-sulphuric acid leaching has been greatly hampered and limited by the need to generate the SO2.
In recent years research work carried out on acid minewaters from coal mines in eastern U.S. has proven the existence of iron-oxidizing autotrophic bacteria which oxidize ferrous ion to ferric much faster than can be done by atmospheric aeration. This strain of bacteria has been tentatively named Thiobacillus ferroxidans.
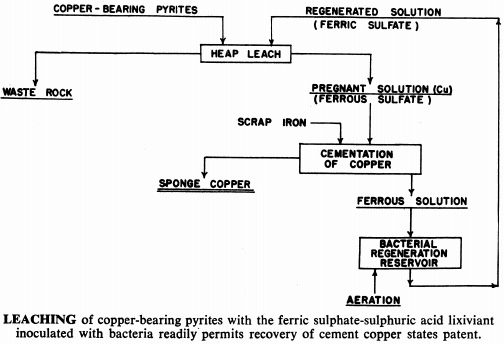
Developing Bacteria Strains
More recently, research by Kennecott Copper on acid mine waters at Bingham Canyon and Chino has proven the existence of similar strains of iron-oxidizing autotrophic bacteria. And a definite indication of the ability of these bacteria to put many metal sulphides into solution, in addition to making the ferrous-ferric conversion, was discovered.
Kennecott found that these strains naturally occurring in the mine water can withstand and grow in relatively high concentrations of dissolved copper ion while having low tolerances for certain other metal ions. For example, Bingham Canyon bacteria will not grow in solutions containing more than 150 ppm zinc even though they will survive and thrive in solutions containing a higher concentration of copper.
By breeding successive generations of these bacteria in culture media more concentrated in other dissolved metals, Kennecott produced new strains of bacteria which will tolerate solutions containing relatively large concentrations of such other metals. For example, bacteria have been developed which have a zinc tolerance as high as 17 grams/liter (17,000 ppm as compared to only 150 ppm in the parent strain). Also, the tolerance to copper has been increased to about 12,000 ppm. Kennecott feels that these tolerances can be increased even more.
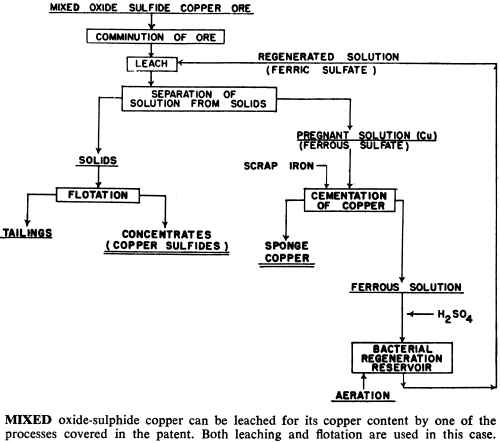
Particular strains of bacteria have been developed to these metal tolerances:
Aluminum………………………………………6,290 ppm
Calcium…………………………………………4,975
Magnesium……………………………………2,400
Manganese……………………………………3,280
Molybdenum…………………………………160
Work has shown that the bacteria can act directly on pyrite to produce ferric sulphate and sulphuric acid. But, even more important, states the patent, is the exceptionally rapid manner in which the bacteria-carrying spent solution can be regenerated industrially by mere aeration without troublesome and expensive apparatus and procedures.
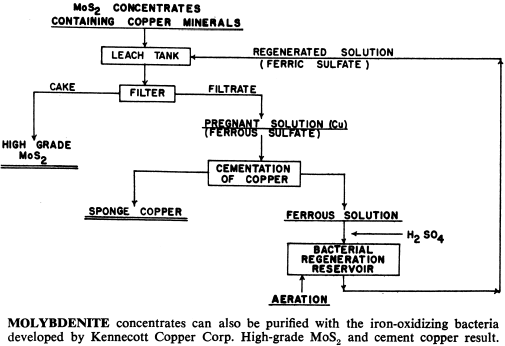
What Processes Have Been Developed
Kennecott’s basic process is a cyclic one using ferric sulphate-sulphuric acid carrying cultures of suitably tolerant bacteria in a sulphide mineral leaching stage which then passes through a regeneration stage within which the bacteria-carrying solution is subject to aeration and recycled to the process.
Repeated heap or vat leaching, or continuous vat leaching in series can be used. Or any other leaching technique may be employed which does not introduce killing amounts of other metal ions into the lixiviant. By aeration, the patent means the introduction of the air and carbon dioxide needed by the bacteria.
Kennecott’s bacteria is a motile, non-sporing rod type approximately 0.5 to 1.0 µ wide and 1.0 to 2.0 µ long. They are autotropic, deriving their energy from the oxidation of ferric ion, and using carbon dioxide as a carbon source. Organic materials are not needed for their subsistence. They require an acid medium. Kennecott’s strain has been deposited with the American Type Culture Collection, 2112 M Street, NW, Washington 7, D.C. It has been given Collection Catalog No. 12912.
Kennecott is sure that similar bacteria can be found in almost any acid mine water. Kennecott has tried leaching with cultured batches added to the leach liquor, but says that it is more advantageous to use naturally occurring bacteria, and increase the metal ion tolerance by gradually increasing the amount of ion in the mine water; but being careful not to wipe out the bacteria in the inoculation process.
Tests have shown that naturally occurring bacteria can thrive in a pregnant solution of copper ion normally used in tin can cementation (11.74 gm/l). Reaction equation for the regeneration of the lixiviant is stated as:
2FeSO4 + H2SO4 + ½ O2 – bacteria → Fe2(SO4)3 + H2O
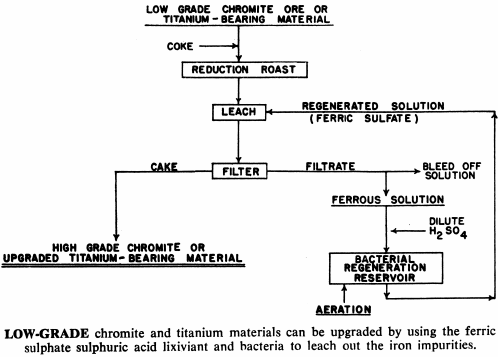
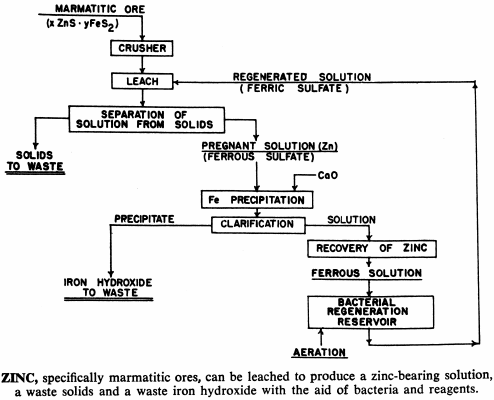
And in cases where the acid is all used up by the leaching process without a corresponding decrease in iron content, the ferric sulphate produced above may hydrolyze, precipitating the ferric salt and liberating more acid.
Such hydrolysis can be valuable if there is enough iron present. Where insufficient iron is available, sulphuric acid may have to be added to protect the ion make-up of the solution.
Iron may be added in the form of pyrite. Iron may be bled off the solution circuit where necessary. Kennecott warns that the equipment must be made of raw materials that will not kill the bacteria in themselves—such as red wood launders, tanks, etc.
Temperature must be controlled so as not to kill or immobilize the bacteria. Kennecott suggests a practical operating temperature maximum of 40 deg C.
Optimum pH in the Kennecott research appears to be 2, but excellent results have been obtained with pH range of 1.5 to 2.5. Bacterial activity decreases progressively and significantly below 1.5 pH.
Laboratory work has shown the possibility of enhancing bacterial action by adding certain nutrients to the lixiviant. Nitrogen in the form of nitrate or ammonium ion increases initial rate of activity, but apparently not affecting the later stages of the reaction. Bacterial activity increased significantly with increased nitrogen additions from 0 to 200 ppm, but economic optimum appears to be 100 ppm. However, after six months of operation, unfed and nitrogen fed bacteria showed the same degree of activation on new sulphide ore.—Source U.S. Patent No. 2,829,964, dated April 8, 1958. Inventors Stuart R. Zimmerley, Dean G. Wilson and John D. Prater, assigned to Kennecott Copper Corp.
Texaco Patents Instant Oxide Leach Process
A patent has been granted to Peter L. Paull, Norwalk, Conn. and assigned to Texaco Development Corp., N. Y., N.Y. that uses heat, pressure, ammonia and carbon dioxide in such a manner as to instantaneously pulverize and leach oxide minerals from low grade ores. Patent No. 2,847,300 specifically mentions such oxidized minerals as malachite, smithsonite, calamine and hydrozincite.
Basically the process is one of heating the ore slurry to high temperature under high pressure, then releasing the pressure to disintegrate the ore particles while at the same time adding ammonia and carbon dioxide to react with the minerals.
One example from the patent will suffice: a low grade zinc ore containing 4.21% smithsonite was crushed to 14 mesh, slurried with water and pumped at 505 psi through 400 ft of ½-in. iron pipe at 935 lb/hour feed rate to an opposed nozzle grinder having 5/32-in. orifice. Outlet pipe temperature was 900 deg F, and downstream pressure was 32 psi. Particles disintegrated to minus 325 mesh. Ammonia and carbon dioxide were injected at the pressure release point and all the zinc minerals went into solution. Solution was filtered, and the zinc precipitated as hydrous zinc carbonate assaying 57% zinc and giving a 93% recovery of the contained zinc.
