As stated above, the Scorification Assay process is especially applicable to (a) complex ores, (b) very rich ores, (c) ores which are mainly valuable for their silver contents. The losses are small and the operations are easy to conduct, and need not be varied much for different classes of ore. For these reasons the process is preferred to the crucible process by some assayers ; if the ore is poor several assays are made and the lead buttons scorified together. The chief disadvantage of the process lies in the small quantity of ore that can be treated, so that the presence of one or two metallic particles of gold may cause the result to be erroneous.
Scorification is conducted in a muffle at a much higher temperature than that required for cupellation. It must be high enough to melt litharge when contaminated by silica and oxides of copper, iron, manganese, &c. A temperature of 1,050° to 1,100° C. is usually enough. The charge is placed in a scorifier, a shallow circular fireclay dish 2 to 3 inches in diameter, which is charged in by the tongs shown at B, Fig. 94. The charge consists of about 50 grains of ore (or 1/10 A.T.), 500 to 1,000 grains of granulated lead, and a few grains of borax glass. The ore is mixed with half the granulated lead, the mixture put in the scorifier and smoothed down, the rest of the lead spread over evenly and the borax put on the top. An addition of some litharge to the cover is often made. The amount of lead to be used varies with the nature of the ore. Ricketts and Miller give the following table as a guide :—
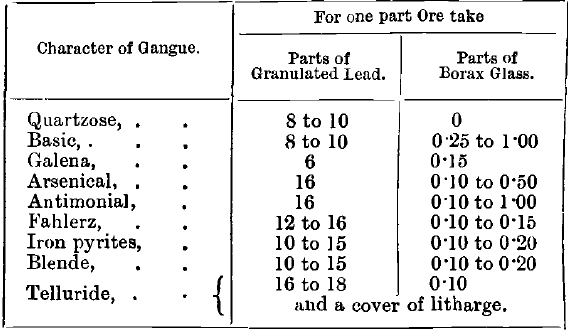
The borax lessens the corrosion of the scorifier and renders the slag more liquid, but its quantity is kept as low as possible to prevent the slag from completely covering the bath of metal too soon. After charging-in, the door of the muffle is closed until fusion takes place. As soon as the lead is melted the door is opened, and a current of air allowed to pass over the bath of metal. Some of the ore is now seen to be floating on the surface of the lead, and is rapidly oxidised, partly by the air and partly by the litharge which immediately begins to form. The sulphur, arsenic, antimony, &c., are thus soon eliminated, while copper, iron, and other bases oxidise and slag off with the borax, and the silica and other acids form fusible compounds with the litharge. Effervescence and spirting may occur, especially if the scorifier has not been well dried by warming before it is used. The slag soon forms a ring completely encircling the bath of metal. As oxidation of lead proceeds, the litharge flows to the sides and increases the quantity of slag until at length the ring closes completely over the metal, leaving a flat uniform surface. This usually happens after from thirty to forty minutes. The slag should be “ cleaned ” before withdrawal. This is done by placing 3 grains of charcoal powder wrapped in tissue paper on the surface of the slag, with a pair of cupel tongs, and closing the muffle door. A number of globules of lead are formed by the reduction of the litharge, and these, falling through the slag, extract and carry down with them any gold and silver which it may still contain, and concentrate them in the molten lead below. The fusion being quiet again, the charge is poured into an iron mould by the scorifying tongs, and the lead button cleaned from slag by a hammer. If the button is too large to cupel at once it is re-scorified in the same dish, fresh lead being added if it is not soft and malleable.
The slag from the scorifier should always be separately retreated in the case of very rich ores, but seldom contains much gold.
The losses incurred in this process are chiefly due to improper temperatures. If the muffle is too cold at first, gold is retained by the slag. This initial low temperature is often indicated by the occurrence of white patches of sulphate of lead on the surface of the slag after pouring. If the slag is pasty, borax is added, but the slag is then rich. It is better to begin again with less ore or more lead. When extremely rich sulphides are assayed, results are often obtained which do not agree well. Stetefeldt recommends in all such cases that the sulphides be attacked by nitric acid and the residues dried and scorified.
Lodge states that the losses of gold in scorifying are greater than in cupelling. He accordingly recommends the use of the former method only when absolutely necessary, as in the assay of zinc residues from the cyanide process, and of copper ingots or bars and material rich in copper. No experiments seem to be on record showing the relative losses of gold when the crucible and scorification methods are used on ordinary quartzose or pyritic ores.
Tellurium ores often yield erroneous results, usually ascribed to volatilisation of the gold, but according to the author’s experience, the vanished gold can be found in the slag or cupel, and has not been volatilised. The tellurium must be driven off before cupellation, as otherwise the gold button may be subdivided into minute particles on the cupel, and in any case tellurium will carry much gold into the cupel.
Lodge recommends the mixture in the scorifier to be covered with litharge when rich tellurides are being assayed. He considers the pot assay and scorification assay to be equally good for tellurides. Tindall and Fulton are in favour of the crucible method.
