Table of Contents
Nickel and cobalt are closely related in their chemical properties, and may best be considered together. Nickel is the commoner of the two, and is met with in commerce alloyed with copper and zinc as German silver; as also in the coinage of the United States and on the Continent. It is used for plating polished iron and steel goods, forming a coating little liable to rust and taking a good polish. The ores of nickel are not very common. Kupfer-nickel and chloanthite are arsenides of nickel with, generally, more or less iron and cobalt. Noumeite and garnierite are hydrated silicates of nickel and magnesia. The chief sources of nickel are these silicates, which are found in large quantity in New Caledonia; and a pyrites found in Norway, containing three or four per cent, of the metal. In smaller quantities it is more widely distributed, being frequently met with in copper ores; consequently, commercial copper is rarely free from it.
Nickel is readily soluble in moderately concentrated nitric acid. Its salts are mostly green, and soluble in excess of ammonia, forming blue solutions; in these respects it resembles copper. The acid solutions, however, are not precipitated by sulphuretted hydrogen, although in alkaline solutions a black sulphide is formed which is insoluble in dilute hydrochloric acid. If the sulphide is formed in a solution containing much free ammonia, the precipitation is incomplete, some sulphide remaining in the solution and colouring it dark brown. These reactions serve to distinguish and separate nickel from other metals, except cobalt. If the separated sulphide be heated in a borax bead, the colour obtained will be a sherry brown in the outer flame, and grey or colourless in the inner flame if nickel only is present. In the presence of cobalt these colours are masked by the intense and characteristic blue yielded in both flames by that metal.
DRY ASSAY
The dry assay of nickel (cobalt being at the same time determined) is based on the formation of a speise which will carry the cobalt, nickel, copper, and some of the iron of the ore in combination with arsenic. A speise of this kind, fused and exposed at a red heat to air, first loses arsenide of iron by oxidation. It is only when the iron has been oxidised that the arsenide of cobalt begins to be attacked; and when the removal of the cobalt is complete, the nickel commences to pass into the slag, the copper being left till last. The changes are rendered evident by fusion in contact with borax. The process is as follows :—Weigh up 5 grams of the ore, and calcine thoroughly on a roasting dish in the muffle. Rub up with some anthracite, and re-roast. Mix intimately with from 3 to 5 grams of metallic arsenic, and heat in a small covered clay crucible at dull redness in a muffle until no more fumes of arsenic come off (about 15 minutes). Take out the crucible, and inject a mixture of 20 grams of carbonate of soda, 5 grams of flour, and 2 grams of fused borax. Place in the wind furnace, and raise the temperature gradually until the charge is in a state of tranquil fusion. Pour; when cold, detach the button of speise, and weigh.
Weigh out carefully a portion of about 1 gram of it. Place a shallow clay dish in the muffle, and heat it to bright redness; then add about 1.5 gram of borax glass wrapped in a piece of tissue paper; when this has fused, drop the piece of speise into it. Close the muffle until the speise has melted, which should be almost at once. The arsenide of iron will oxidise first, and when this has ceased the surface of the button brightens. Remove it from the muffle, and quench in water as soon as the button has solidified. The borax should be coloured slightly blue. Weigh: the loss is the arsenide of iron. Repeat the operation with the weighed button on another dish, using rather less borax. Continue the scorification until a film, green when cold, floating on the surface of the button shows that the nickel is beginning to oxidise. Cool, separate, and weigh the button as before. The loss is the arsenide of cobalt.
If copper is absent, the speise is now arsenide of nickel.
The weight of nickel corresponding to the arsenide got is calculated by multiplying by 0.607; and, similarly, the weight of the cobalt is ascertained by multiplying the loss in the last scorification by 0.615. It must be remembered that the nickel and cobalt so obtained are derived from a fraction only of the speise yielded by the ore taken, so that the results must be multiplied by the weight of the whole of the speise, and divided by the weight of the fragment used in the determination. As an example, suppose 5 grams of ore gave 3.3 grams of speise, and 1.1 gram of this gave 0.8 gram of nickel arsenide. Then—
0.8 x 0.607 = 0.4856 gram of nickel
0.4856 x 3.3 ÷ 1.1 = 1.456 gram of nickel
And this being obtained from 5 grams of ore is equivalent to 29.12 per cent.
When copper is also present, weigh up accurately about 0.5 gram of gold, and place it on the scorifier with the button of nickel and copper arsenide, using borax as before. Scorify until the button shows the bluish-green colour of a fused gold-copper alloy. Then cool, and weigh the button of copper and gold. The increase in weight of the gold button gives the copper as metal The weight of the copper multiplied by 1.395 is weight of the copper arsenide (Cu3As) present. The difference will be the nickel arsenide.
The student should enter the weighings in his book as follows :

WET METHODS
Solution and Separation
Two or three grams of a rich ore, or 5 to 10 grams if poor, are taken for the assay. If much arsenic is present (as is usually the case), the ore must be calcined before attacking with acids. Transfer to a flask; and boil, first with hydrochloric acid until the oxides are dissolved, and then with the help of nitric acid, until nothing metalliferous is left. Dilute, nearly neutralise with soda, and separate the iron as basic acetate, as described in page 233. Through the filtrate pass sulphuretted hydrogen till saturated. Allow to settle (best over-night), filter, and wash. Transfer the precipitate to a beaker, and dissolve in nitric acid. Dilute with water, pass sulphuretted hydrogen, and filter off the precipitate, if any. Boil off the gas, add ammonia until a precipitate is formed, and then acidify somewhat strongly with acetic acid. Pass sulphuretted hydrogen in a slow stream until any white precipitate of zinc sulphide, there may be, begins to darken. Filter; to the filtrate add ammonia, and pass sulphuretted hydrogen. The precipitate will contain the nickel and cobalt as sulphides.
Where small quantities of nickel and cobalt are present, and an approximate determination is sufficient, they can be concentrated as follows:—Remove the copper, &c., by passing sulphuretted hydrogen through the acid solution and filtering; add ammonia to the filtrate, and again pass sulphuretted hydrogen; then heat nearly to boiling, and filter. Dissolve the precipitate off the filter with dilute hydrochloric acid ; the residue will contain nearly all the nickel and cobalt as sulphides.
Separation of Nickel and Cobalt
Dissolve the sulphides separated as above in nitric acid; render alkaline with a solution of potash, then acidify with acetic acid ; add a concentrated solution of nitrite of potash. The liquid after this addition must have an acid reaction. Allow to stand for 24 hours in a warm place. Filter off the yellow precipitate of nitrite of potash and cobalt, and wash with a 10 per cent, solution of acetate of potash. The cobalt is determined in the precipitate in the way described under Cobalt. The nickel is separated from the solution by boiling with sodic hydrate, filtering, and dissolving the precipitate in nitric acid. The solution will contain the nickel.
GRAVIMETRIC DETERMINATION
The solution, which contains the nickel free from other metals, is heated, and a solution of sodic hydrate added in slight excess. The precipitate is filtered off, washed with boiling water, dried, ignited at a red heat, and weighed when cold. The ignited substance is nickel oxide (NiO), and contains 78.67 per cent, of nickel. The oxide is a green powder, readily and completely soluble in hydrochloric acid, and without action on litmus paper. It is very easily reduced by ignition in hydrogen to metallic nickel.
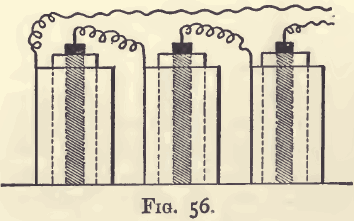 Nickel is also determined by electrolysis, as follows:—The nitric acid solution is rendered strongly ammoniacal, and placed under the electrolytic apparatus used for the copper assay. Three cells (fig. 56),
Nickel is also determined by electrolysis, as follows:—The nitric acid solution is rendered strongly ammoniacal, and placed under the electrolytic apparatus used for the copper assay. Three cells (fig. 56),
however, must be used, coupled up for intensity, that is, with the zinc of one connected with the copper of the next. The electrolysis is allowed to go on over-night, and in the morning the nickel will be deposited as a bright and coherent film. A portion of the solution is drawn off with a pipette; if it smells of ammonia, has no blue colour, and gives no precipitate with ammonic sulphide, the separation is complete. Wash the cylinder containing the deposited metal, first with water and then with alcohol, as in the copper assay. Dry in the water oven, and weigh. The increase in weight is metallic nickel.
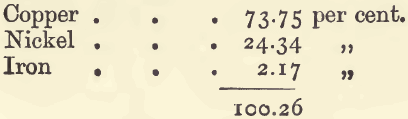
As an example:—There was taken 1 gram of a nickel alloy used for coinage. It was dissolved in 10 c.c. of nitric acid, and diluted to 100 c.c. with water. The copper was then precipitated by electrolysis. It weighed 0.734 gram. The solution, after electrolysis, was treated with sulphuretted hydrogen, and the remaining copper was thrown down as sulphide, and estimated colorimetrically. This amounted to 3½ milligrams. The filtrate was evaporated, treated with ammonia, warmed, and filtered. The ferric hydrate was dissolved in dilute acid, and reprecipitated, dried, ignited, and weighed. Its weight was 0.0310 gram. The two filtrates were mixed, and reduced in bulk to about 50 c.c.; a considerable excess of ammonia was added, and the nickel precipitated by electrolysis. It weighed 0.2434 gram. These quantities are equivalent to:
VOLUMETRIC DETERMINATION
An alkaline solution of potassium cyanide, to which a little potassium iodide has been added, can be assayed for its strength in cyanide by titrating with a standard solution of silver nitrate. Nickel interferes with this assay, doing the work of its equivalent of silver; and the quantity of nickel present can be calculated from the amount of its interference in the titration. A volumetric assay for nickel is based on this. It has the disadvantage of all indirect titrations in that it requires two standard solutions. On the other hand it gives good results even under unfavourable conditions, and is applicable in the presence of much zinc. Small quantities of cobalt will count as so much nickel, but larger quantities make the assay unworkable. Some of the other metals— lead for example—have no appreciable effect; but practically the solution demands a preliminary treatment which would result in their removal. Nevertheless it is a very satisfactory method and makes the determination of nickel quick and comparatively easy in most cases. The standard solution of silver nitrate is made by dissolving 14.48 grams of recrystallised silver nitrate in distilled water and diluting to 1 litre : 100 c.c. of this solution are equivalent to 0.25 gram of nickel.
The standard solution of potassium cyanide should be made so as to be exactly equal to the silver nitrate solution. This can be done as follows: Weigh up 12 grams of good potassium cyanide (95 Per cent.), dissolve in water, add 50 c.c. of a 10 per cent, solution of sodium hydrate and dilute to 1 litre. Fill one burette with this and another with the solution of silver nitrate. Run 50 c.c, of the cyanide into a flask; add a few drops of potassium iodide solution and titrate with the standard silver nitrate until there is a distinct permanent yellowish turbidity. The titration is more fully described under Cyanide, p. 165. The cyanide solution will be found rather stronger than the silver nitrate; dilute it so as to get the two solutions of equal value. For example, 51.3 c.c. of silver nitrate may have been required: then add 1.3 c.c. of water to each 50 c.c. of the cyanide solution remaining. If the full 950 c.c. are available, then add to them 24.7 c.c. of water. After mixing, take another 50 c.c. and titrate with the silver nitrate; the two solutions should now be exactly equal. The cyanide solution, being strongly alkaline with soda, keeps very well; but its strength should be checked from time to time by titrating with silver nitrate; should there be any slight inequality in the strengths of the two solutions it is easily allowed for in the calculations.
Titration
The solution, containing not much more than 0.1 gram of nickel, and free from the interfering metals, must be cooled. It is next neutralised and then made strongly alkaline with a solution of soda (NaHO); an excess of 20 or 30 c.c. suffices. This will produce a precipitate. The cyanide solution is now run in from a burette until the solution clears, after which an excess of about 20 c.c. is added. It is well to use some round number of c.c. to simplify the calculation. Add a few drops of potassium iodide solution, and run in the standard solution of silver nitrate from a burette. This should be done a little at a time, though somewhat rapidly, and with constant shaking, till a permanent yellow precipitate appears. If the addition of the cyanide did not result in a perfectly clear solution, this is because something besides nickel is present. The residue may be filtered off, though with a little practice the finishing-point may be detected with certainty in the presence of a small precipitate. If the student has the slightest doubt about a finish he should run in another 5 c.c. of the cyanide and again finish with silver nitrate. The second result will be the same as the first. For example, if 40 c.c. of cyanide and 30 c.c. of silver nitrate were required at the first titration, then the 45 c.c. of cyanide in the second titration will require 35 c.c. of silver nitrate. The difference between the quantities of the two solutions used in each case will be 10 c.c. It is this difference in the readings of the two burettes which measures the quantity of nickel present. Each c.c. of the difference is equal to .0025 gram of nickel. But if the cyanide solution is not exactly equal in strength to the silver nitrate, the quantity of cyanide used should be calculated to its equivalent in silver nitrate before making the subtraction.
The following experimental results illustrate the accuracy of the assay and the effect upon it of varying conditions. A solution containing 1 gram of nickel sulphate (NiSO4.6H2O) in 100 c.c. was used. By a separate assay the sulphate was found to contain 22.25 per cent, of nickel. For the sake of simplicity the results of the experiments are stated in weights of nickel in grams.
Effect of varying excess of Cyanide Solution
In each experiment there was 20 c.c. of the nickel solution, equal to .0445 gram of nickel. There were also 10 c.c. of soda solution, 3 or 4 drops of potassium iodide and sufficient water to bring the bulk to 100 c.c. before titrating.
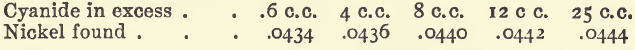
Although the difference between the highest and lowest of these results is only 1 milligram, their meaning is quite obvious. The excess of cyanide should not be less than 20 c.c.
Effect of varying the quantity of Soda
There were two series of experiments, one with 2 c.c. of nickel solution (= .0044 gram of nickel), the other with 20 c.c. The conditions were as before, except that the quantity of soda was varied.

These show that the presence of much soda, though it has only a small effect, is beneficial rather than otherwise. Ammonia has a bad effect, if present in anything like the same quantities.
Effect of varying the Nickel
In experiments with 10, 20, and 40 c.c. of the nickel solution, the results were:—
![]()
Effect of Zinc
In these experiments 20 c.c. of nickel solution (=.0445 gram of nickel), 10 c.c. of soda, 6 drops of potassium iodide and water to 100 c.c. were used. The excess of cyanide was purposely kept at from 10 to 15 c.c., which is hardly sufficient.
![]()
On increasing the excess of cyanide to over 20 c.c. and doubling the quantity of soda, the experiment with 0.5 gram of zinc gave 0.441 gram of nickel. Hence the titration is satisfactory in the presence of zinc provided that not fewer than 20 or 30 c.c. of soda are used, and that the excess of cyanide is such that not fewer than 20 or 30 c.c. of silver nitrate are required in the titration. Moreover, these precautions should be taken whether zinc is present or not.
Effect of other Metals on Nickel Assays
If metals of the first and second groups are present they should be removed by passing sulphuretted hydrogen and filtering. If iron is present it must be removed, since ferrous salts use up much cyanide, forming ferrocyanides, and ferric salts yield ferric hydrate, which obscures the end reaction. Hence the sulphuretted hydrogen must be boiled off and the iron removed as basic ferric acetate by the method described on p. 233. If the precipitate is bulky it should be dissolved in a little dilute acid, neutralised and again precipitated as basic acetate. The nickel will be in the two filtrates. In the absence of manganese and cobalt the titration may be made without further separation.
Manganese does not directly interfere, but the precipitated hydrate, which rapidly darkens through atmospheric oxidation, obscures the end reaction. It may be removed by passing sulphuretted hydrogen through the filtrate from the acetate separation : sulphides of nickel cobalt and zinc will be precipitated, whilst manganese remains in solution: the addition of more sodium acetate may assist the precipitation. The precipitate must be filtered off and dissolved in nitric acid : the solution should be evaporated to dryness. The filtrate may retain a little nickel; if so, add ammonia till alkaline, then acidify with acetic acid and again filter; any small precipitate obtained here should be added to that first obtained.
It is only when cobalt is present that any further separation is required. Cobalt hydrate takes up oxygen from the air, and on adding potassium cyanide some may refuse to dissolve; and the solution itself acquires a brown colour, which becomes deeper on standing. At this stage the cobalt is easily separated. The solution containing the nickel and cobalt with no great excess of acid, is made alkaline by adding 20 c.c. of soda exactly as in preparing for a titration. So, too, the solution of cyanide is added so as to have an excess of 20 or 30 c.c.; the solution may have a brown colour, but if it is not quite clear it must be filtered. Then warm (boiling is not needed) and add from 50 to 100 c.c. of bromine water. This throws down all the nickel as black peroxide in a condition easy to filter. Filter it off and wash with water. The precipitate can be dissolved off the filter with the greatest ease by a little warm sulphurous acid. The filtrate and washings, boiled till free from sulphurous acid, yield the nickel as sulphate in a clean condition.
Determination of Nickel in Nickel Sulphate Crystals
Take 0.5 gram of the salt, dissolve in 50 c.c. of water and add 25 c.c. of solution of soda. Run in from a burette, say, 60 c.c. “ cyanide.” Add a few drops of potassium iodide and titrate back with “ silver nitrate.” Suppose 15.5 c.c. of the latter is required. Then 15.5 c.c. subtracted from 60 c.c. leaves 44.5 c.c., and since 100 c.c. = 0.25 gram of nickel, 44.5 c.c. will equal 0.11125 gram of nickel. This in 0.5 gram of the salt equals 22.25 per cent.
Determination of Nickel in German Silver
Weigh up 0.5 gram of the alloy, and dissolve in a dish with 5 or 10 c.c. of dilute nitric acid. Add 5 c.c. of dilute sulphuric acid and evaporate till all the nitric acid is removed. Cool, take up with 50 c.c. of water, and when dissolved pass sulphuretted hydrogen through the solution. Filter off the precipitate and wash with water containing sulphuretted hydrogen and dilute sulphuric acid. Boil down the filtrate and washings to get rid of the excess of the gas; add some nitric acid and continue the boiling. Cool, neutralise the excess of acid with soda, add 1 gram of sodium acetate and boil. Filter off the precipitate which contains the iron. The filtrate, cooled and rendered alkaline with soda, is ready for the titration.
