Table of Contents
- Dry Assay
- Scorification Assay
- POT ASSAYS
- Determination of Silver in Assay Lead
- Determination of Silver in Red Lead or Litharge
- Determination of Silver in Argentiferous Lead
- Determination of Silver in Bullion
- Determination of Silver in Copper
- Determination of Silver in Galena
- Determination of Silver in an Ore
- Determination of Silver in Silver Precipitate
- WET METHODS
- GRAVIMETRIC DETERMINATION
- Determination of Silver in Burnt Ores
- Determination of Silver in Commercial Copper
- VOLUMETRIC METHODS
- SULPHOCYANATE METHOD
- COLORIMETRIC DETERMINATION
Silver is widely diffused, and has been found in most mining districts. It occurs native in sufficient quantity to constitute one of the chief ores of the metal. It also occurs combined with sulphur (as in argentite), with sulphur and antimony (as in stephanite or brittle silver ore, and in pyrargyrite or ruby silver), and with copper, sulphur, antimony, and arsenic, as in polybasite. Chloride of silver occurs native as horn silver or kerargyrite. Silver is found in the ores of other metals, such as fahlerz, which sometimes contains from two to ten per cent, of the metal, and galena, which is an important source of it; in fact, galena is never found entirely free from silver. It is present also in greater or less quantity in the ores of copper and zinc.
Silver dissolves readily in nitric acid, forming silver nitrate It only forms one family of salts, and of these the chloride and nitrate are of chief importance to the assayer. The formation of the chloride of silver on the addition of hydrochloric acid or a soluble chloride to the nitric acid solution, serves for the recognition and separation of silver. The precipitated chloride is white (becoming violet on exposure to light), insoluble in nitric acid, soluble in ammonia, hyposulphite of soda, or concentrated solutions of chlorides. The best confirmatory test is made by wrapping the precipitate in a little sheet lead, and cupelling, when the silver will be left in the metallic state, and is easily recognized.
Dry Assay
This assay is made up of two parts: (1) the concentration of the silver in a button of lead; and (2) the cupellation of the resulting alloy. The concentration of the button of lead may be effected either by scorification or by fusion in a crucible.
The scorification assay is performed in a scorifier, which is a shallow open-mouthed dish about 2½ inches across, with a very thick bottom to enable it to withstand the corrosive action of the slag. A charge of more than 3 or 5 grams of the ore cannot be worked in one, and with such small charges the unavoidable variations have a serious effect on the figures reported. A difference of one milligram on the weight of the button of silver got represents a difference of 6 or 10 ounces per ton. With rich ores such variation is unavoidable under any conditions, and the only safe plan is to take the mean of several assays. But with poorer ores the accuracy of the assay, as well as convenience in working, is much increased by working in a crucible with larger charges.
In scorification the proportion of lead required for scorifying 1 gram of ore is in average cases from 10 to 15 grams, sinking in the case of galena to 2 grams, and rising with earthy and refractory substances to from 30 to 40 grams. But by fusing in a crucible with well-selected fluxes, a proportion of 4 of flux to 1 of ore is generally sufficient; and not only is the proportion of added matter less, but it is also easier to manipulate large quantities in crucibles, so that, although in some cases the crucible assay is more troublesome and less satisfactory, yet with poor and earthy ores it is the best method of dealing with them ; while when properly worked it yields results as accurate as scorification does. As a general rule, if more than 5 grams of ore must be taken, the crucible assay should be adopted.
Scorification Assay
The charge of ore is usually 3 grams, sometimes 5 ; the lead varies from 30 to 70 grams, and the quantity of soda, borax, or powdered glass added varies from 0.3 to 3 or 4 grams. It is generally recommended to have the lead granulated, and to mix the ore with about half of it in the scorifier; then to put on the rest of the lead; and finally to sprinkle the borax or glass on the top. It answers just as well, however, to use the lead in the shape of foil, and wrap the ore up in it; and if the ore contains much sulphur, the borax may with advantage be added (wrapped in a little tissue paper) some
five or ten minutes after the operation has started.
The process of scorification is as follows :—A scorifier (fig. 38) of convenient size having been selected (one 21 inches across is most generally useful), it is dried at a gentle heat for about ten minutes.

The charge is then put into it, and it is introduced, with the help of a scorifier tongs (fig. 39), into a muffle heated considerably above redness. The muffle is then closed, and when the metal has melted down, it is opened, but the temperature is kept up. A ring of slag will, after a time, form around the metal, and when this appearance (known as the eye) presents itself, the temperature may be lowered. When the eye has disappeared—that is, when the layer of slag has quite closed in—a pinch of powdered culm wrapped in tissue paper is added. As soon as the slag has again become tranquil, the scorifier is taken out, and its contents are poured into a mould (fig. 40), the slag is detached, and saved. If the button of metal weighs more than 30 grams, its size is reduced by another scorification in the same scorifier, which should have been replaced in the muffle immediately after the contents had been poured out. If the ore is not a very rich one, the button of lead will carry practically all the silver; but with rich ores it is more satisfactory to save the slag, and subsequently to melt it down with the cupel on which the lead has been treated, so as to recover the silver lost in the slag, together with that absorbed in the cupel, at one operation. Or, if the cupellation loss is neglected or calculated in some other manner, the slag or slags from the scorifier may be powdered and mixed with 20 grams of oxide of lead, 5 grams of borax, and 1 gram of charcoal. This should be melted down in a small crucible, and the resulting button of lead cupelled.
If the scorification has been unsatisfactory, the quantity of silver obtained from the slag will be by no means inconsiderable. The usual explanation is that with sulphury ores compounds of metallic oxides and sulphides (oxysulphides) are formed, which remain in the slag, retaining considerable quantities of the precious metal. It is said that under certain conditions such a slag may contain as much as 10 per cent, of silver. An excess of lead and a high temperature prevents the formation of these oxysulphides. But if much silver is present in the ore, the slag cannot be safely thrown away, even if sulphur is absent, and the process has been satisfactorily performed.
If the crust which appears on the surface of the lead does not clear, add a small lump, of borax and 20 grams more lead ; then close the muffle, and keep the temperature as high as possible. If the slag forms properly, but shows unfused or only half-fused lumps, even when the scorification has proceeded for some time, add more borax, and stir with an iron rod. The slag adhering to the rod must be detached by hammering, and replaced in the scorifier.
If the ore consists largely of quartz, soda should be added instead of borax; or, if it contains much copper, powdered quartz may be used. If the scorifier at the end of an operation is more than usually corroded, the borax should be replaced in subsequent assays on similar ores by powdered glass or quartz.
If a fairly fluid slag is formed which does not clear from the metal and show the eye, more lead and a higher temperature is wanted.
As a general rule, it may be stated that when a scorification is unsatisfactory, what is wanted is more heat, more lead, or more borax.
It is a safe plan when work has to be done on a strange ore, to make three or four assays with varying quantities of lead. The proportion of lead is right when a further addition does not yield a higher result. The proper proportion having been found, a note of it should be made for future use.
POT ASSAYS
The object of the fusion in a crucible, like that of scorification, is to concentrate the silver in a button of lead which is to be subsequently cupelled; and to retain the earthy and waste matters in the slag. It is necessary to consider the quality of the slag and the weight and quality of the lead. The slag when fused should be liquid and homogeneous, and not too corrosive on the crucible. The button of lead should be soft, malleable, and free from a coating of regulus. In weight it should not differ much from the ore taken. With 20 grams of ore, for example, a button of lead weighing from 18 to 25 grams will be satisfactory : less than this would leave an undue proportion of silver in the slag; and more would be unnecessarily large for cupelling, and would increase the loss in that operation.
With average ores, take 20 grams of the powdered ore and mix with 30 grams of “ soda,” 40 grams of red-lead or litharge, 5 grams of borax, and from 2 to 2.5 grams of flour, and place in an E crucible (Battersea round). Put these in the furnace at a red heat, cover the crucible, and gradually raise the temperature until the whole charge has melted down and is in a state of tranquil fusion. Pour into a mould, and replace the crucible in the furnace. As soon as the lead is solid, detach the slag and put it back into the crucible ; and when it is again fluid, charge on to it with a copper scoop a mixture of 20 grams of oxide of lead, and 1 gram of charcoal : when fusion has again become tranquil, pour and detach the button of lead. The lead buttons should be hammered into discs with rounded edges, and be freed from slag ; if too big for a cupel they may be scorified together in a small scorifier, but it is better to cupel them separately.
Assaying Ores containing Metallic Oxides
Peroxides of iron, manganese, and copper interfere by counteracting the effect of the charcoal or flour, and thus reducing the size of the lead button. Peroxide of iron will reduce the weight of lead by a little more than its own weight; and peroxide of manganese has about twice this effect. When these oxides are present an additional quantity of flour must be used, and precautions must be taken to prevent re¬oxidation of the slag by the furnace gases. This may best be prevented by using a layer of common salt as a cover to the charge. When the ores contain a good deal of quartz or stony matter, the fluxes just given (for average ores) will do; but the proportion of soda should be diminished, and that of the borax, oxide of lead, and flour increased as the quantity of metallic oxides become greater. If the ore contains practically no quartz, the soda may be altogether omitted, and some glass or powdered quartz added. The following charge may be taken as an example: weigh up 20 grams of the powdered ore, 15 grams each of “ soda” and borax, 60 grams of oxide of lead, and 5 grams of flour. Mix and place them in an E crucible, and cover with a layer of from a quarter to half an inch of common salt. Place in the furnace as before. The salt will give off a considerable amount of fume, which will, to a certain extent, conceal the state of the charge: when the crucible has been in the furnace for about 25 minutes remove it and pour out the contents immediately. With ores that produce a thick slag the addition of 5 grams of fluor spar will be an advantage. It may happen that with an unknown ore the first assay will be more or less unsatisfactory : but from it the necessity for adding more or less flour will be learnt, and a second assay, with the necessary modification of the charge, should give a good result.
Assaying Ores containing much Sulphides
Ores of this class may be easily recognized, either by the appearance of the minerals they contain or by the odour of sulphurous oxide (SO2) which they evolve when roasted on a spatula. The sulphides most commonly present, in addition to the sulphurized minerals of silver, are pyrites, galena, blende, and mispickel. When they are present in only a moderate amount, their effect is simply to increase the weight of the button of lead; and this is easily counteracted by reducing the amount of flour, or by omitting it. When in larger amounts, they not only yield large buttons, but also render the metal sulphury, sometimes even giving a button of regulus instead of lead. This last evil may be remedied (1) by putting in a rod of iron as soon as the charge has fused, or (2) it may be counteracted by a proper addition of nitre, or (3) when the sulphides present are only those of iron or copper the sulphur may be removed by calcining, and the ore converted into one of the class containing metallic oxides. The calcination is effected as follows :—Weigh up 20 grams of the powdered ore and place it in a wide-mouthed crucible sufficiently large to perform the subsequent melting down in. The roasting must be done at a gentle heat at first, so as to avoid clotting : the mouth of the crucible should project considerably above the coke, and should slope forward towards the worker. The charge must be occasionally stirred with the stirrer (fig. 10) so as to expose fresh surfaces to the action of the air, and to prevent adhesion to the sides of the crucible. The stirrer should not be removed till the calcination is finished. The temperature should be raised at the end to a good red heat; and (to ensure the decomposition of any sulphate that may be formed) the roasted ore should be rubbed up in a mortar with a pinch of anthracite, and again calcined. It is then mixed with fluxes as described, and fused in the same crucible.
The calcination of an ore is a work occupying a good deal of time, and, in most cases, it is better to take advantage of the desulphurizing power of red lead or nitre. Red lead by itself will do, but a large quantity of it will be required; 1 part of a metallic sulphide needs from 20 to 50 parts of red lead to yield a button free from sulphur; whereas at most from 2 to 2½ parts of nitre are sufficient. There is sometimes an advantage in having a considerable excess of oxide of lead in the slag, but where there is no such reason, 2 parts of red lead to 1 of ore is enough. A charge which will do for most sulphides is the following: 20 grams of ore, 40 to 100 grames of red lead, 20 grams of “ soda,” 5 of borax, and sufficient nitre (or perhaps flour) to give a button of about 25 grams of lead. How much this must be (if not already known) may be approximately determined by fusing 3 grams of the ore and 3 grams of “ soda ” in a small crucible (C) with 50 grams of litharge (not red lead) under a cover of salt, and weighing the resulting button of lead. Subtract 3 from the weight of lead obtained, and the difference multiplied by 1.3 will give the quantity in grams of nitre required. If the button of lead weighs less than 3 grams flour must be added. If this is not satisfactory repeat the assay, adding an extra gram of nitre for each 4 grams of lead in excess of that required, or 1 gram of flour for a 12-gram deficiency.
In the method in which iron is used as a de-sulphurising agent, only as much oxide of lead should be added as will give a button of lead of the required size. Rather a large button of lead should be got, and the slag should be strongly alkaline; if the ore does not already carry a large amount of sulphur some should be added. The fusion should be performed at a low temperature (similar to that for a galena assay), and should be continued for some time after it has become tranquil. Take 20 grams of the ore, 40 grams of “soda,” 40 grams of oxide of lead, and 5 or 10 grams of borax; place this mixture in a crucible (with a rod of iron, as in the galena assay), cover, and fuse for about half an hour. Take out the rod, washing it in the slag, and, in a minute or two, pour. Clean and cupel the button of lead.
General Remarks on the Fusion
Other things being equal, the smaller the quantity of the slag the better, provided there is sufficient to cover the metal. The presence of peroxides of the heavy metals is prejudicial, since they tend to increase the quantity of silver retained in the slag. It may be given as a general rule that when iron, copper, manganese, &c., are present, there is a more than ordinary need for cleaning the slags, and care must be taken to keep these metals in the state of lower oxide.
In selecting the fluxes, it should be remembered that soda is the best for quartz, and borax for lime and metallic oxides. And that with ores almost free from gangue some quartz or glass should be added to protect the crucible. Two parts of soda are enough to flux 1 part of quartz ; whilst of borax, or oxide of lead, 4 parts are barely sufficient. Oxide of lead has the advantage of being heavy and so does not occupy much space in the crucible ; on the other hand, if the melting down be performed too quickly, or if oxide of lead only is used, this high specific gravity is a disadvantage, for the lighter earthy matter floats as a pasty mass on the more fluid oxide of lead, and thus escapes its action.
When metallic sulphides are present in the ore, an excess of oxide of lead helps to keep the sulphur out of the button of metal. In addition to the oxide of lead required as a flux, some will be required to provide the lead in which the silver is to be collected. Oxide of lead, mixed with charcoal or flour, yields, when heated, a multitude of minute buttons of metal uniformly distributed through the mass of the charge; as the charge melts down these run together and fall to the bottom; this shower of lead collects the silver more easily than a single button at the bottom of the crucible could do. Only that portion of the oxide of lead which remains in the slag can be considered as a flux; very often the first indication of an excessive reduction of lead is the pastiness of the slag rendered thick by the withdrawal of the oxide of lead which would have kept it fluid. If, in an assay, it is found that 5 parts of flux are not sufficient for 1 part of ore, the remedy lies in using a different flux rather than in taking a larger quantity.
On the Reducing Effect of Charcoal, Flour, and Tartar.—The weight to be got from a given charge will depend (provided sufficient oxide of lead is present) upon the proportion of the reducing agents in it. We have thought it well to illustrate this part of the subject by a series of experiments which the learner will do well to practise for himself before proceeding to the assay of actual ores. Take 80 grams of litharge and 20 grams of a mixture of borax and soda. Fuse three lots (1) with 1.5 gram of charcoal, (2) with 3 grams of flour, and (3) with 7.5 grams of tartar. Weigh the buttons of lead obtained, and divide each by the weight of reducing agent used. The results will differ somewhat with the dryness and quality of the flour, etc., used; in one series of experiments they were as follows:—

The use of flour as a reducing agent has many advantages, and it is well to remember that 1 gram of flour reduces about 11 grams of lead; and that charcoal has twice, and tartar one-half, this reducing effect.
On the Reducing Effect of Charcoal, &c., on Red Lead.—It is often easier to obtain red lead of good quality than it is litharge, and by a large number of assayers red lead is the form of oxide of lead always used. Red lead, however, contains an excess of oxygen which will use up some of the reducing agent before lead separates out. On making a series of experiments (similar to the last, but using 80 grams of red lead instead of the litharge) the results were, with the same quantities of the reducing agents:—
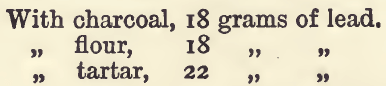
Comparing these with the results with litharge, in the previous table it will be seen that the same quantity of reducing agent has in each case brought down 16 grams less of lead, so that a larger amount of the reducing agent must be added to get a button of the same weight as that obtained with litharge. To get a button of a desired weight, say 22 grams, we must add reducing agent sufficient to throw down 22 + 16 or 38 grams of lead, which would require 3.4 grams of flour. If this amount of flour is fused with 80 grams of red lead, a button of lead weighing 22 grams will be formed, the other 16 grams being kept up by the oxygen of the red lead.
If the quantity of red lead differs from 80 grams, this rule must be modified. With 40 grams of red lead, for example, we should add an excess of reducing agent sufficient to throw down 8 grams of lead instead of 16. Similarly, with 160 grams of red lead, we should add enough to throw down 32 grams.
The following rule will enable one to calculate the weight of flour required to produce a button of lead of any desired weight from any given quantity of red lead. Each 5 grams of red lead present diminishes the weight of the lead by 1 gram. If then we divide the weight of red lead in a charge by 5, and add this to the weight of lead required, the sum divided by 11 will give the weight of flour which must be added. Using 80 grams of red lead and wanting a button of 20 grams, we should add 3.3 grams of flour.
80 ÷ 5 = 16; 16 + 20 = 36; 36 ÷ 11 = 3.3 nearly
The following are some results obtained which will illustrate the rule :—

On the Reducing Effect of Metallic Sulphides, and the Counteracting Effect of Nitre.—The sulphides found in ores will reduce a button of lead from oxide of lead just as flour does; and, as charcoal, flour and tartar differ in their reducing power, so equal weights of the different mineral sulphides throw down different weights of lead.
One gram of iron pyrites yields about 11 grams of lead. One gram of copper pyrites, blende, fahlerz, or mispickel, yields 7 or 8 grams of lead, whilst 1 gram of antimonite will give 6, and 1 gram of galena only a little over 3 grams. It is evident that if an ore carries much of these sulphides, the quantity of lead reduced will be very much larger than that required for an assay. To counteract this effect nitre is added; 1 gram is added for each 4 grams of lead in excess of that required. For example : with a 20-gram charge of an ore containing 50 per cent, of pyrites, if no nitre were added, 110 grams of lead would be got; or, if there was not sufficient oxide of lead to yield this quantity of metal, the button would be sulphury. To reduce the weight of the button by 80 grammes, we should add 20 grams of nitre, if litharge were used; or if red lead were used, we should add 16 grams of nitre, since the oxidizing effect of 20 grams of red lead is equivalent to that of 1 of nitre, and since 80 grams of red lead are generally used in a charge. Two assays of an ore of this kind with these quantities of nitre gave 26.0 grams of lead with litharge, and 22.5 grams with red lead.
It is best to use in these assays 80 grams of red lead, 20 of soda, and 5 of borax, with 20 grams of the ore. If the lead got by the preliminary fusion in a small crucible with litharge (described under “ ores containing much sulphides ”) is known, the following table will indicate the quantity of nitre, or flour, to be added with this charge :—
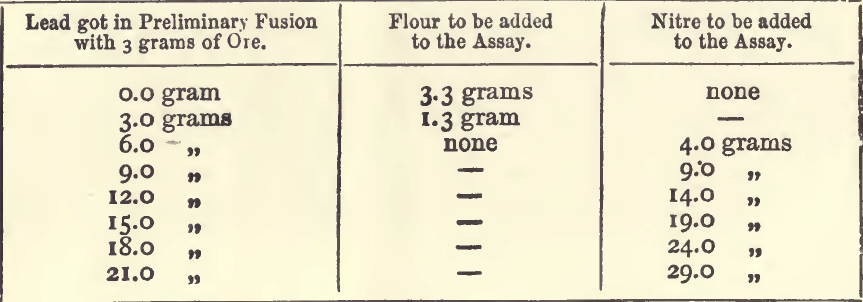
If litharge is used in the assay instead of red lead 4 grams more nitre, or 1.5 gram less flour must be used. When more than a few grams of nitre are added to a charge the proportion of “soda” and borax should be increased, because one of the products of the reaction of nitre upon sulphides in the presence of soda is sulphate of soda, and because the “ soda ” thus used up no longer serves as a flux; more borax should be added, as it is the best flux for the metallic oxides which are formed in the process. If in an assay too large a button of lead is got, even after this calculation has been made, and the assay is repeated, add 1 gram more nitre for each 4 grams of lead in excess. Sometimes the assay appears tranquil before the nitre has produced its full effect; in such cases it is well to seize the crucible with the tongs and mix its fused contents by rotating them ; if this causes an effervescence, the crucible should be replaced in the fire and the fusion continued. The following experiments will illustrate the extent to which the above rules may be relied on. In all of them the standard flux was used, viz.:—80 grams of red lead, 20 of soda, and 5 of borax.
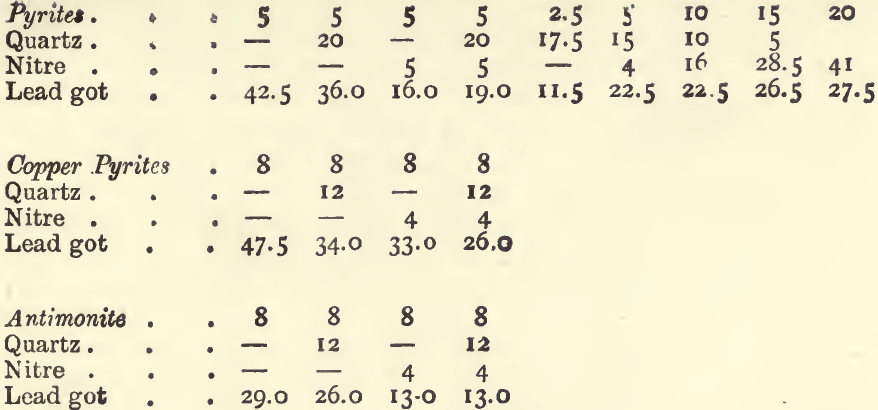
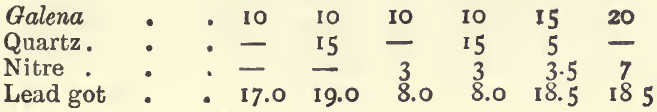
A similar set of experiments, with 80 grams of litharge instead of 80 grams of red lead, gave :—
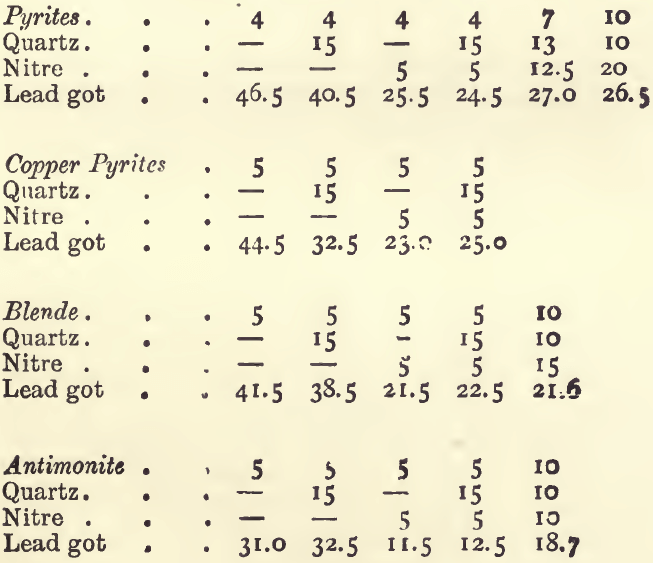
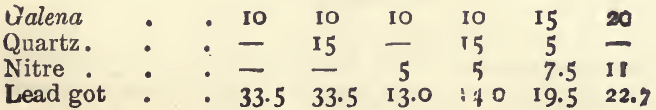
The variation in some of these experiments, in which we might have expected similar results, is due to the fact that the sulphur, and in some cases the metals, are capable of two degrees of oxidation. For example : theoretically 1 gram of iron pyrites (FeS2) would yield 8.6 grams of lead if the sulphur were oxidised to sulphurous oxide (SO2), and the iron to ferrous oxide (FeO); whilst if the sulphur were oxidised to sulphate (SO3), and the iron to ferric oxide, 12.9 grams of lead will be thrown down. Similarly the yield with copper pyrites would be 7.5 or 11.6 ; with blende, 6.4 or 8.5 ; with antimonite, 5.5 or 8 ; and with galena, 2.6 or 3.4. As regards the metals, the lower oxide will always be formed if the assay is carried out properly (fused under a cover, and with a sufficiency of reducing agent). But the proportion of sulphur oxidised completely will vary with the conditions of the assay. With a slag containing much soda the tendency will be to form sulphate, and, in consequence, a big reduction of lead ; whilst with an acid slag containing much quartz the tendency will be for the sulphur to go off as sulphurous oxide (SO2). In a fusion with litharge alone all the sulphur will be liberated as the lower oxide, whilst with, much soda it will be wholly converted into sulphate. For example : 3 grams of an ore containing a good deal of pyrites and a little galena, gave, when fused with litharge, 16.5 grams of lead. A similar charge, containing in addition 20.0 grams of soda, gave 22.5 grams of lead.
It will be noted from the experiments that 1 gram of nitre kept up on the average 4 grams of lead ; the range being from 3.2 with acid slags to 5.3 with very basic ones. These facts serve to explain some apparently irregular results got in practice.
https://www.911metallurgist.com/cupellation-silver-assaying
Determination of Silver in Assay Lead
Scorify 50 grams of the lead with 0.5 gram of powdered quartz or glass at not too high a temperature. When the eye has “ closed in,” pour; reject the slag, and cupel the button of lead. Remove the cupel from the muffle immediately the operation is finished. Weigh, and make a prominent note of the result in the assay book, as so many miligrams of silver contained in 100 grams of lead.
Determination of Silver in Red Lead or Litharge
Fuse 100 grams of the oxide with from 10 to 20 grams of borax; and in the case of litharge with 2 grams or with red lead 4 grams of flour. Cupel the lead, and weigh the button of silver. Note the result as in the last case.
Determination of Silver in Argentiferous Lead
Be careful in taking the sample, since with rich silver lead alloys the error from bad sampling may amount to several parts per cent. Cupel two lots of 20 grams each, and weigh the buttons of silver. Add to these the estimated cupel loss, and calculate the result. Or wrap each button of silver in 20 grams of assay lead, and re-cupel side by side with two fresh lots of 20 grams each of the alloy. Calculate the loss incurred, and add on to the weight of the two fresh buttons got.
Determination of Silver in Bullion
The remarks made tinder the last heading as to the importance of correct sampling apply with equal force here. Make a preliminary assay by cupelling 0.1 gram of the alloy with 1 gram of assay lead; calculate the percentage composition. Refer to the table on page 105 to find what weight of lead is required for cupelling 1 gram of alloy.
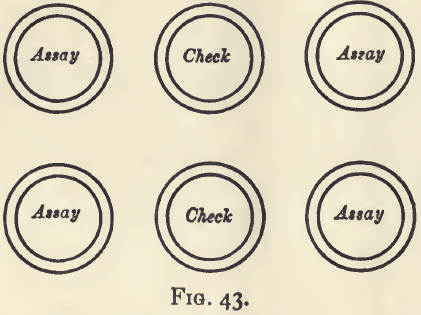
Weigh out four lots of 1 gram each, and wrap them in the required quantity of lead. Make two check pieces by weighing up two lots of fine silver equal to that which you believe to be present in the assay pieces ; add copper to make up the weight to 1 gram, and wrap in the same quantity of lead as was used for the assays.
Prepare six cupels and charge them in the annexed order (fig. 43), and cupel. Guard against spirting. Clean and weigh the buttons of silver. Add the mean loss on the two check pieces to the mean weight of the four assay pieces; this multiplied by 1000 will give the degree of fineness.
Determination of Silver in Copper
The silver is best separated in the wet way before cupelling, but if the proportion is not too small, it can be found by cupellation. Weigh up 3 grams of the metal, wrap in 30 grams of sheet lead, and cupel; when the cupellation has proceeded for fifteen minutes, add 20 grams more lead, and continue till finished. Weigh the button of silver.
The cupellation loss will be five or six per cent, of the silver present. Determine it by powdering the saturated portion of the cupel and fusing in a large Cornish crucible with 30 grams each of soda and borax, 10 grams of fluor spar, and 1½ gram of charcoal. Cupel the resulting button of lead, and add 10 grams more of lead towards the close of the operation. Deduct the weight of silver contained in the lead used from the weight of the two buttons, and calculate to ounces to the ton.
In an experiment in which 0.1975 gram of silver was present, the weight of the button from the first cupellation was 0.1867, and that of the button from the second, after correcting for the lead added, was 0.0110 gram.
Determination of Silver in Galena
By Pot Assay.— Mix 20 grams of the powdered ore with 30 grams of red lead, 20 grams of soda, and 5 grams of borax, as also with from 7 to 10 grams of nitre. Fuse and pour. Clean the slag if the ore is rich. Cupel the buttons of lead. Make the usual corrections and calculate in ounces to the ton.
By Scorification.—Take 10 grams of the ore, 30 grams of lead, and 0.5 gram of borax. Scorify, clean the slag by adding anthracite after the “ eye ” has closed in : cupel the button of lead. Weigh the button of silver, make the necessary corrections, and calculate to ounces to the ton.
The determination may also be made by cupelling the button of lead got in the dry lead assay.
A sample of galena determined by the three methods gave tho following results:—

Determination of Silver in an Ore
By Pot Assay.—Take 20 grams of the powdered ore and mix with 30 grams of soda, 40 grams of red lead, and 5 grams of borax, as also with from 2 to 3 grams of, flour. Fuse : pour. Clean the slag by fusing with 20 grams of red lead and two grams of flour. Cupel the buttons of lead ; weigh ; make the necessary corrections, and calculate to ounces to the ton.
By Scorification.—Take 5 grams of the powdered ore, 50 grams of lead, and 0.5 gram of “ soda ” or borax. Scorify. Clean the slag by fusing in a crucible as in the pot assay. Cupel, &c.
Determination of Silver in Silver Precipitate
This substance contains, in addition to metallic silver and gold, sulphates of lead and lime; oxides of zinc, copper, and iron; and more or less organic matter. The sample as received is generally free from “ water at 1oo° C.”; and, since it rapidly absorbs water, care should be taken in weighing it.
Since it contains combined water it is not suited for scorifying ; therefore the determination of silver and gold (fine metal) is made by pot assay. Weigh up 5 grams of the precipitate, mix with 100 grams of litharge and 1 gram of charcoal. Melt in a crucible at a moderate heat and pour. Detach the slag, replace in the crucible, and, when fused, add a mixture of 20 grams of litharge and 1 gram of charcoal. When the fusion is again tranquil, pour; and cupel the two buttons of lead.
In a sample worked in this manner the mean of four determinations gave 0.6819 gram of “fine metal”; deducting 1 milligram for the silver contained in the oxide of lead, and adding 8 milligrams for the cupellation loss, there is got 0.6889 gram or 13.778 per cent of silver (and gold) in the sample.
Determination of Silver in Burnt Ores. By Pot Assay. —Roasted cupriferous pyrites containing small quantities of gold and silver comes under this heading. The following mixture will give a fluid slag which is heavy and tough when cold :—

Mix; place in a large crucible; cover with salt; and melt down under cover. When fused drop in an iron rod for a few minutes, and about a couple of minutes after its withdrawal, pour the charge quickly into a large conical mould. The button of lead should weigh about 50 grams. Cupel and weigh the silver. The litharge may be replaced by red lead, in which case another gram of charcoal powder must be added.
In our experience the results obtained by this method are about 20 per cent, less than the actual content of the ore. The results of two assays, after deducting for the silver in the litharge used, were 3.9 and 4.1 milligrams; and a third assay, in which 5.4 milligrams of silver had been added, gave 9.2, which, after deducting the added silver, leaves 3.8 milligrams. The average of the three results is 3.9 milligrams from the 100 grams of ore.
Two lots of 100 grams of the same ore treated in the wet way gave 5.2 and 5.0 milligrams of silver. Burnt ores from Spanish pyrites carry about 0.005 per cent, of silver.
WET METHODS
Silver is got into solution from its ores by attacking with nitric acid, but it is best, after dissolving, to cautiously add dilute hydrochloric acid, and to carefully avoid excess. If the quantity of silver is very small the solution is allowed to stand twenty-four hours, but, otherwise, it is warmed and filtered as soon as it clears.
Dry the residue and concentrate the silver in a button of lead by pot method or scorification, according to the amount of stony matter present. Cupel the lead, and the resulting button will be free from all metals, except perhaps gold. It may be weighed; or dissolved in nitric acid, and the silver determined gravimetrically in the diluted and filtered solution. It is better to weigh the metal and afterwards to determine the gold in it, estimating the silver by difference. Silver alloys are dissolved in dilute nitric acid (free from chlorides), diluted, and filtered. The solution is then ready for gravimetiic determination.
Sulphuretted hydrogen precipitates silver (like copper), completely, even from fairly acid solutions.
GRAVIMETRIC DETERMINATION
Add dilute hydrochloric acid in small excess to the hot dilute solution, which must contain free nitric acid. Heat and stir until the solution clears. Decant through a small filter, and wash with hot water, acidulated at first with a little nitric acid if bismuth is suspected to be present. Dry quickly, transfer as much as possible of the precipitate to a watchglass ; burn and ignite the filter paper, treating the ash first with two drops of nitric acid and then with one of hydrochloric, and again dry. Add the rest of the silver chloride and heat slowly over a Bunsen burner until it begins to fuse. Cool and weigh.
The precipitate is silver chloride (AgCl) and contains 75.27 per cent, of silver. The moist precipitate is heavy and curdy; it is decomposed by direct sunlight, becoming violet under its influence. When heated it is yellowish; and, since it is volatile at a high temperature, it must not, in drying, be heated above its fusing point. The fused chloride can be removed from the crucible (to which it adheres strongly) by digesting with dilute acid and zinc.
For the determination of silver in nearly pure bullion the following process is used:—Weigh up 1.5054 gram of the alloy. With this amount of alloy each 2 milligrams of silver chloride formed is equivalent to 1 degree of fineness, so that the weight of the silver chloride obtained (stated in milligrams and divided by 2) will give the degree of fineness. Transfer to a bottle (known as “ bottles for the Indian mint assay ”) and dissolve in 10 c.c. of dilute nitric acid, then make up with water to 200 c.c. and add 3 c.c. of dilute hydrochloric acid. Allow to stand a few minutes and then shake. Fill the bottle completely with water, allow to settle, and syphon off the clear liquid; pour on more water, shake gently to break up the lumps, and again fill the bottle with water. Invert over the mouth of the bottle a porous Wedgwood crucible, somewhat similar to those used in gold parting. Take firm hold of the crucible and bottle, and invert promptly so that the silver chloride may be collected in the crucible. Allow to stand a little while for the precipitate to settle, and then carefully remove the crucible under water. Drain off most of the water and break up the silver chloride with the help of a well-rounded glass rod. This greatly facilitates the subsequent drying. Dry first on the water bath and then on the iron plate. Remove the dried silver chloride, by inverting the crucible, and weigh it.
As an example, 3 determinations of silver in a coin carried out in this way gave ;—
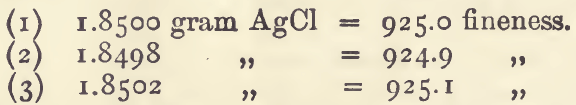
Determination of Silver in Burnt Ores
Take 100 grams of the ore and place in a large beaker of 2½ litres capacity, and cover with 375 c.c. of hydrochloric acid. Boil for half an hour until the oxides are dissolved and the residue looks like sand and pyrites; then add 20 c.c. of nitric acid, and boil till free from nitrous fumes. Dilute to 2 litres with water, and pass a current of sulphuretted hydrogen till the iron is reduced, the copper and silver precipitated, and the liquor smells of the gas. This takes about one hour and a half.
Filter off the precipitate (rejecting the solution) and wash with warm water. Dry and transfer to an evaporating dish, adding the ashes of the filter paper. Heat gently with a Bunsen burner until the sulphur burns, and then calcine until no more sulphurous oxide comes off. When cold add 30 c.c. of nitric acid, boil and dilute to 100 c.c. Add 1 c.c. of very dilute hydrochloric acid (1 to 100), stir well, and allow to stand overnight. Decant on to a Swedish filter paper, dry and calcine.
Mix the ashes with 100 grams of litharge and 1 gram of charcoal, and fuse in a small crucible. Detach the button of lead and cupel. Weigh and make the usual corrections. As an example, 100 grams of ore treated in this way gave 5.8 milligrams of silver; deducting 0.8 for the silver added in the oxide of lead leaves 5 milligrams obtained from the ore. Another experiment on 100 grams of the same ore to which 5 milligrams of silver had been added gave 11.0 milligrams. Deduct 5.8 for the silver added; this leaves 5.2 milligrams as the silver obtained from the ore. These give, as a mean result, 0.0051 per cent., or 1.66 ounce per ton.
Determination of Silver in Commercial Copper
For the method of doing this, with an example and experiment, see under the heading of Examination of Commercial Copper.
VOLUMETRIC METHODS
There are two of these, one adapted for the determination of silver in alloys of approximately known composition, and the other of more general application. The first of these, generally known as “ Gay-Lussac’s ” method is, as regards its working, perfect in principle ; but it requires a practically constant quantity of silver, that is, one which varies by a few milligrams only in each determination. It is a confirmatory method rather than a determinative one. The other is known as “ Volhard’s,” and resembles in principle and method an ordinary volumetric process.
Gay-Lussac’s method is based on the precipitation of silver from a nitric acid solution by a solution of sodium chloride. The point at which the whole of the silver is precipitated being recognised by the standard solution ceasing to give a precipitate. The process depends for its success upon, (1) the ease which silver chloride separates out from the solution leaving it clear after shaking, and, (2), the cloudiness produced by the reaction of very small quantities of silver nitrate and sodium chloride. In working, a quantity of the sodium chloride solution equal to 1 gram of silver is added at once to the assay; and, when the solution has been rendered clear by shaking, the residual silver (which should not exceed a few milligrams) is estimated with the help of a weaker solution of sodium chloride. The success in working evidently depends upon the accuracy with which the first addition of the salt solution is made. On this account the standard solution is run in from a special pipette capable of delivering a practically invariable volume of solution. It is not so important that this shall deliver exactly 100 c.c. as that in two consecutive deliveries the volume shall not differ by more than 0.05 c.c. The dilute salt solution is one-tenth of the strength of that first run in, and 1 c.c. of it is equivalent to 1 milligram of silver. Ordinarily it is run in 1 c.c. at a time (and an ordinary burette may be used for this purpose), shaking between each addition until it ceases to give a precipitate. If many such additions have to be made the operation not only becomes tedious, but the solution also ceases to clear after shaking, so that it becomes impossible to determine the finishing point.
If the assay contains less than one gram of silver the first addition of the dilute salt solution of course produces no precipitate. Five milligrams of silver an solution (5 c.c.) is then added, and the assay proceeded with in the usual way; 5 milligrams of silver being deducted from the amount found.
There is required for the assay a standard solution of sodium chloride, which is prepared by dissolving 5.4162 grams of the salt (made by neutralizing carbonate of soda with hydrochloric acid) in water and diluting to one litre. 100 c.c. of this is equivalent to 1 gram of silver.
The weaker solution of salt is made by diluting 100 c.c. of the stronger one to one litre. One c.c. of this will equal 1 milligram of silver, or 0.1 c.c. of the stronger solution.
A standard solution of silver equivalent to the dilute salt solution is made by dissolving 1 gram of fine silver in 10 c.c. of dilute nitric acid, and diluting with water to one litre.
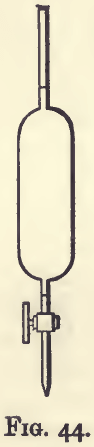
The solution of salt is standardised as follows :—Weigh up 1.003 gram of fine silver and dissolve in 25 c.c. of dilute nitric acid in a bottle provided with a well-fitting flat headed stopper. Heat on the water bath to assist solution, resting the bottle in an inclined position. When dissolved blow out the nitrous fumes with the help of a glass tube bent at right angles. Run in from a stoppered pipette (as shown in fig. 44) 100 c.c. of the standard salt solution, and shake vigorously until the solution clears. Fill an ordinary burette with the weaker standard salt solution, and run 1 c.c. into the assay bottle, letting it run down the side so that it forms a layer resting on the assay solution.
If any silver remains in solution a cloudy layer will be formed at the junction where the two liquids meet. This is best observed against a black background. If a cloudiness is seen, shake, to clear the liquid, and run in another c.c. of salt, and continue this until a cloudiness is no longer visible. Deduct 1.5 c.c. from the amount of the weaker sodium chloride solution run in. Divide the corrected reading by 10, and add to the 100 c.c. This will give the volume of strong salt solution equivalent to the silver taken.
If the first addition of the weaker salt solution causes no cloudiness add 5 c.c. of the silver solution from an ordinary pipette, shake, and then run in the weaker salt solution, working as before. These 5 milligrams of silver added must be allowed for before calculating. As an example:—1.0100 gram of fine silver was taken for standardising a solution and 4 c.c. of the weaker salt solution were run in. Deducting 1.5 and dividing by 10 gives 0.25 c.c. to be added to the 100 c.c.
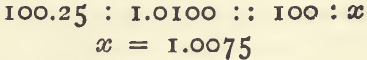
which is the standard of the salt solution.
The method of working an assay may be gathered from the following example:—In the determination of silver in some buttons left after cupellation, it was assumed that these would contain 99.5 per cent, of silver. For the assay it was necessary to take a quantity that should contain a little more than 1.0075 grams silver; then

To ensure a slight excess, there was taken 1.0150 gram of the buttons, which was treated in exactly the same way as for the standardising. The quantity of the weaker salt solution required was 7 c.c.; deducting 1.5 c.c., and dividing by 10, gives 100.55 c.c. of strong salt solution, which is equivalent to 1.0130 gram of silver. This being obtained from 1.015 gram of alloy, is equal to 99.8 per cent., or 998.0 fine.
Effect of Temperature
The standardising and the assay must be done at the same time, since a difference of 5° C. makes a difference of 0.1 c.c. in measuring the 100 c.c. of strong solution of salt. It is always best to prepare a standard with each batch of assays.
SULPHOCYANATE METHOD
Volhard’s process is based upon the precipitation of silver in nitric acid solutions with potassium sulphocyanate, the finishing point being the development of a reddish-brown colour, produced by the action of the excess of sulphocyanate upon ferric sulphate. The white sulphocyanate settles readily, leaving the liquor clear and a persistent brown coloration in the liquid indicates the finish. The assay must be carried out in the cold ; and water free from chlorides must be used.
The standard sulphocyanate of potassium solution is made by dissolving 4½ or 5 grams of the salt (KCyS) in water, and diluting to 1 litre. 100 c.c. are about equivalent to 0.5 gram of silver.
The standard silver nitrate solution is made by dissolving 5 grams of fine silver in 50 c.c. of dilute nitric acid, boiling off nitrous fumes, and diluting to 1 litre.
The indicator is a saturated solution of iron alum, or a solution of ferric sulphate of equivalent strength made by titrating acid ferrous sulphate with potassium permanganate. Use 2 c.c. for each assay.
The sulphocyanate solution is standardised by placing 50 c.c. of the silver nitrate solution in a flask with 20 c.c. of dilute nitric acid, diluting to 100 c.c. with water, and running in the sulphocyanate until the greater part of the silver is precipitated; then adding 2 c.c. of the ferric indicator, and continuing the titration until a reddish-brown colour is developed, and remains permanent after shaking continuously. The assay is similarly performed, the silver being used in the state of a nitric acid solution.
The effect of variations in the conditions of the assay may be seen from the following experiments, in which 20 c.c. of standard silver nitrate were used :—
Effect of Varying Temperature :—
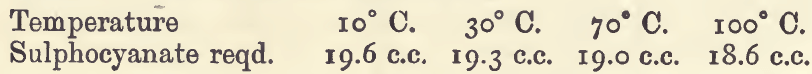
Effect of Varying Nitric Acid:—Varying nitric acid has no effect, except that with a fairly acid solution the finishing point is somewhat sharper.
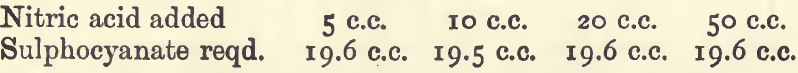
Effect of Varying Bulk:—
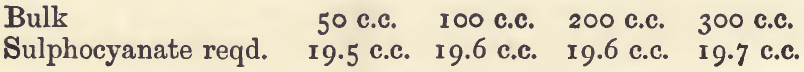
Effect of Varying Ammonic Nitrate: —
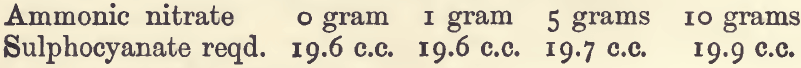
Effect of Varying Silver:—
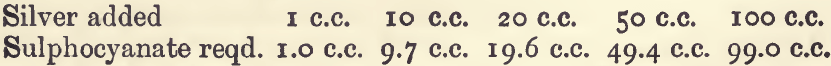
This method is valuable for determining silver in salts, alloys, and solutions, where no more than an ordinary degree of accuracy is demanded. It is easy, and applicable under most of the usual conditions. Its greatest disadvantage is the brown coloration produced by the sulphocyanate when the assay is nearly, but not quite, finished; and the slowness with which this is removed on shaking up with the precipitate. This is worse with large quantities of precipitate, and if about 1 gram of silver is present, it gives an indefiniteness to the finish which lowers the precision of the process to about 1 in 500; this is useless for the assays of bullion. One writer states that this inconvenience is due to portions of liquid being entangled in the precipitate, but it appears much more likely to be due to the action of the precipitate itself. In attempting to apply the process to the assay of bullion by working it on the principle of a Gay-Lussac assay, it was found that a very considerable excess of silver was required to complete the reaction. In these experiments 100 c.c. of “sulphocyanate ” (very accurately measured) was run into the solution containing the weighed portion of bullion (fine silver) and, after shaking the solution, was filtered. In the filtrate the remaining silver, if there should be any, was determined by the ordinary titration, but with “ sulphocyanate ” of one tenth the strength. This final titration was quite satisfactory. The amount of silver precipitated by the first 100 c.c., however, varied with the quantity of silver present as in the following series.

These, of course, preclude a method of the kind aimed at, and at the same time emphasise the importance of uniformity of work in the ordinary process. In the determination of chlorides in sea-water, Dittmar used a combined method: precipitating the bulk of the silver as chloride, and after filtering, determining the small excess of silver by sulphocyanate. This modification answers admirably when applied to the assay of bullion. In the ordinary Gay-Lussac method, the precipitation of the bulk of the silver by the 100 c.c. of salt solution leaves nothing to be desired, either as to ease in working or accuracy of result; the silver precipitate settles quickly, and leaves a clear liquor admirably fitted for the determination of the few milligrams of silver remaining in solution. But the method of determining this residual silver by adding successive small quantities of salt so long as they continue to give a precipitate is unsatisfactory, and, judged on its own merits apart from the rest of the process, could hardly escape condemnation. It is clumsy in practice, for the continued adding of small portions of salt solution is laborious and becomes impossible with more than a few milligrams of silver in solution. The proposed modification is simple; having precipitated the silver with the 100 c.c. of salt solution, as described under Gay-Lussac’s method (page 120), shake till the liquor clears, and filter into a flask, washing with a little distilled water. Add 2 c.c. of “ ferric indicator ” to the filtrate and titrate with a standard “ sulphocyanate solution ” made by diluting the ordinary standard solution to such an extent that 100 c.c. after diluting shall be equivalent to 0.1 gram of silver. Calculate the weight of silver found by ‘‘sulphocyanate” and add it to the weight which 100 c.c. of the salt solution will precipitate.
An advantage of this modification is that an excess of 15 milligrams may be determined as easily and exactly as 5. In standardising the salt solution, then, weigh up, say 1.0150 gram of pure silver, dissolve and titrate. Suppose 13.5 c.c. of “ sulphocyanate ” required ; then these are equivalent to .0135 gram of silver, (100 c.c. = .1); the silver precipitated by the salt is 1.0150 – .0135—i.e. 1.0015 gram, which is the standard.
Application of the Method to Assays for Arsenic
If silver nitrate be added to a neutral solution of an arsenate of one of the alkali metals, silver arsenate (Ag3AsO4), is thrown down as a dark-red precipitate. If, after adding excess of silver nitrate to insure a complete precipitation, the arsenate of silver be filtered off, the weight of the arsenic could be estimated from the weight of silver arsenate formed. But this may be done much more conveniently by dissolving the precipitate in nitric acid, and titrating with sulphocyanate; the silver found will be to the arsenic present as 324 (108 x 3) is to 75.
The mineral is best treated by the method given in the third paragraph on page 382 ; but the solution, after being acidified with nitric acid, should be made exactly neutral with ammonia. A small excess of silver nitrate should then be added, and since acid is liberated in the reaction, the liquor must again be neutralised. The precipitate must then be filtered off, and washed with distilled water. Then dissolve it in the paper by slowly running over it 20 c.c. of dilute nitric acid. Wash the filter with distilled water, collecting with the filtrate in a small flask. Add 2 c.c. of “ ferric indicator ” and titrate.
If the sulphocyanate solution be made up with 11 or 12 grams of the potassium salt to the litre, and be then standardised and diluted, so that for 100 c.c. it shall equal 1.08 gram of silver, (see p. 38), then it will also equal .25 gram of arsenic (As). Except for ores rich in arsenic, it will be better to work with a solution one half this strength. The standard as calculated from an experiment with pure silver should be checked by another using pure resublimed white arsenic, As2O3, which contains 75.75 % of the metal. The quantity of white arsenic taken, .1 or .2 gram, should contain about as much arsenic as will be present in the assays. It is converted into sodium arsenate by evaporating to a small bulk with nitric acid and neutralising with soda. The precipitation and titration of the silver arsenate should be exactly as in the assays.
The difficulty of the method is in the neutralising; which has to be very carefully done since silver arsenate is soluble in even faintly acid solutions; one drop of nitric acid in 100 c.c. of water is enough to produce an absolutely worthless result; and an excess of acid much less than this is still very prejudicial. The addition of a little sodium acetate to the solution after the final neutralising has a good effect.
Arsenic in Mispickel
Weigh up .250 gram of the finely powdered ore, and place in a Berlin crucible about 1¼ or 1½ inch in diameter. Treat with 10 or 12 drops, one drop at a time, of strong nitric acid, warm very gently, but avoid much heating. Put on a thin layer of nitre, and rather more than half fill the crucible with a mixture of equal parts of soda and nitre. Heat quickly in the blow-pipe flame, and when the mass is fused and effervescing, withdraw and allow to cool. Boil out with water, filter and wash. Insert a piece of litmus paper and cautiously neutralise with nitric acid, using ammonia to neutralise any accidental excess of the acid. Add a gram or so of ammonium nitrate and silver nitrate in excess, neutralise again with ammonia and add two or three grams of sodium acetate. Filter off the precipitate, wash and titrate. In the fusion care should be taken to avoid much effervescence (an excess of the soda mitigates this) and the operation should be stopped as soon as the whole has entered into fusion.
COLORIMETRIC DETERMINATION
There is, properly speaking, no colorimetric method, but the following, which is sometimes used, is based on similar principles.
It is useful for the determination of small quantities of silver in substances which yield clear solutions with nitric acid.
Dissolve a weighed quantity of the substance in nitric acid, and dilute to a definite bulk. Divide into two equal parts. To one, add a drop or two of dilute hydrochloric acid, stir and filter. To the other, add a similar amount of dilute acid, and then to the filtered portion run in from a burette standard silver nitrate (1 c.c. = 0.5 milligram silver) until the solutions are equally turbid. Calculate in the usual way.
