Acetic Acid, HAc or C2H4O2. (sp. gr. 1.044, containing 33 per cent, real acid).—An organic acid, forming a class of salts, acetates, which are for the most part soluble in water, and which, on ignition, leave the oxide or carbonate of the metal. It is almost always used in those cases where mineral acids are objectionable. To convert, for example, a solution of a substance in hydrochloric acid into a solution of the same in acetic acid, alkali should be added in excess and then acetic acid. Many compounds are insoluble in acetic acid, which are soluble in mineral acids, such as ferric phosphate, ferric arsenate, zinc sulphide, calcium oxalate, &c., so that the use of acetic acid is valuable in some separations. The commercial acid is strong enough for most purposes, and is used without dilution.
“Aqua Regia” is a mixture of 1 part by measure of nitric acid and 3 parts of hydrochloric acid. The acids react forming what is practically a solution of chlorine. The mixture is best made when wanted, and is chiefly used for the solution of gold and platinum and for “opening up” sulphides. When solutions in aqua regia are evaporated, chlorides are left.
Bromine, Br. (sp. gr. 3.0). Practically pure bromine.—It is a heavy reddish-brown liquid and very volatile. It boils at 6o° C., and, consequently, must be kept in a cool place. It gives off brown irritating vapours, which render its use very objectionable. Generally it answers the same purpose as aqua regia, and is employed where the addition of nitric acid to a solution has to be specially avoided. It is also used for dissolving metals only from ores which contain metallic oxides not desired in the solution.
“Bromine Water” is simply bromine shaken up with water till no more is dissolved.
Carbonic Acid, CO2.—A heavy gas, somewhat soluble in water; it is mainly used for providing an atmosphere in which substances may be dissolved, titrated, &c., without fear of oxidation. It is also used in titrating arsenic assays with “iodine” when a feeble acid is required to prevent the absorption of iodine by the alkaline carbonate. It is prepared when wanted in solution, by adding a gram or so of bicarbonate of soda and then as much acid as will decompose the bicarbonate mentioned. When a quantity of the gas is wanted, it is prepared, in an apparatus like that used for sulphuretted hydrogen, by acting on fragments of marble or limestone with dilute hydrochloric acid.
Citric Acid (H3Ci or C6H8O7.H2O) is an organic acid which occurs in colourless crystals, soluble in less than their weight of water. The solution must be freshly prepared, as it gets mouldy when kept. It forms a comparatively unimportant class of salts (citrates). It is used in the determination of phosphoric acid, chiefly for the purpose of preventing the precipitation of phosphates of iron and alumina by ammonia, and in a few similar cases. The commercial crystals are used ; they should be free from sulphuric acid and leave no ash on ignition.
Hydrochloric Acid, HCl in water, (sp. gr. 1.16. It contains 32 per cent, of hydrogen chloride).—It is sometimes called “muriatic acid,” and when impure, “spirit of salt.” The acid solution should be colourless and free from arsenic, iron, and sulphuric acid. It forms an important family of salts, the chlorides. It is the best acid for dissolving metallic oxides and carbonates, and is always used by the assayer when oxidising agents are to be avoided. The acid is used without dilution when no directions are expressly given to dilute it. It has no action on the following metals: gold, platinum, arsenic, and mercury ; it very slightly attacks antimony, bismuth, lead, silver, and copper. Tin is more soluble in it, but with difficulty; whilst iron, zinc, nickel, cobalt, cadmium, and aluminium easily dissolve with evolution of hydrogen and the formation of the lower chloride if the metal forms more than one class of salts. All the metallic oxides, except a few of the native and rarer oxides, are dissolved by it with the formation of chlorides of the metal and water.
Dilute Hydrochloric Acid is made by diluting the strong acid with an equal volume of water. This is used for dissolving precipitates obtained in the general course of analysis and the more easily soluble metals.
Hydrofluoric Acid, HF.—A solution in water may be purchased in gutta-percha or lead bottles. It is of variable strength and doubtful purity. It must always be examined quantitatively for the residue left on evaporation. It is used occasionally for the examination of silicates. It attacks silica, forming fluoride of silicon, which is a gas. When the introduction of another base will not interfere with the assay, the substance may be mixed in the platinum dish with fluoride of ammonium or of potassium, or of calcium, and hydrochloric acid, instead of treating it with the commercial acid. It is only required in special work. The fumes and acid are dangerous, and, of course, glass or porcelain vessels cannot be used with it.
Iodine, I.—This can be obtained in commerce quite pure, and is often used for standardising. It is very slightly soluble in water, but readily dissolves in potassium iodide solution. It closely resembles chlorine and bromine in its properties, and can be used for dissolving metals without, at the same time, attacking any oxide which may be present. It is chiefly used as an oxidizing agent in volumetric work, being sharp in its reactions and easily detected in minute quantities. It cannot be used in alkaline solutions, since it reacts with the hydrates, and even with the carbonates, to form iodides and iodates. Iodine is soluble in alcohol.
Nitric Acid, HNO3. (Sp. gr. 1.42 ; boiling point 121° C.; contains 70 per cent, by weight of hydrogen nitrate).—It is convenient to remember that one c.c. of this contains 1 gram of real acid. It combines the properties of an acid and of an oxidising agent. One c.c. contains 0.76 gram of oxygen, most of which is very loosely held, and easily given up to metals and other oxidisable substances. Consequently it will dissolve many metals, &c., upon which hydrochloric acid has no action. All sulphides (that of mercury excepted) are attacked by it, and for the most part rendered soluble. It has no action on gold or platinum, and very little on aluminium. The strong acid at the ordinary temperature does not act on iron or tin; 2nd in most cases it acts better when diluted. Some nitrates being insoluble in nitric acid, form a protecting coat to the metal which hinders further action. Where the strong acid does act the action is very violent, so that generally it is better to use the dilute acid. When iron has been immersed in strong nitric acid it not only remains unacted on, but assumes a passive state ; so that if, after being wiped, it is then placed in the dilute acid, it will not dissolve. Tin and antimony are converted into insoluble oxides, while the other metals (with the exception of those already mentioned) dissolve as nitrates. During the solution of the metal red fumes are given off, which mainly consist of nitrogen peroxide. The solution is often coloured brown or green because of dissolved oxides of nitrogen, which must be got rid of by boiling. Generally some ammonium nitrate is formed, especially in the cases of zinc, iron, and tin, when these are acted on by cold dilute acid. Sulphur, phosphorus, and arsenic are converted into sulphuric, phosphoric, and arsenic acids respectively, when boiled with the strong acid.
Dilute Nitric Acid.—Dilute 1 volume of the strong acid with 2 of water.
Oxalic Acid, H2O or (H2C2O4.2H2O.)—This is an organic acid in colourless crystals. It forms a family of salts—the oxalates. It is used in standardising; being a crystallised and permanent acid, it can be readily weighed. It is also used in separations, many of the oxalates being insoluble. For general use make a 10 per cent, solution. Use the commercially pure acid. On ignition the acid should leave no residue.
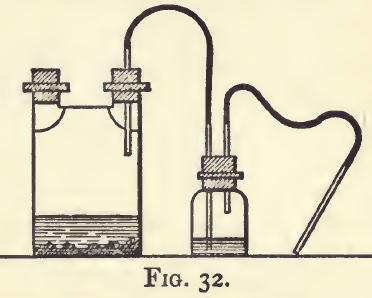
Sulphuretted Hydrogen. Hydrosulphuric acid, SH2.—A gas largely used in assaying, since by its action it allows of the metals being conveniently classed into groups. It is soluble in water, this liquid dissolving at the ordinary temperature about three times its volume of the gas. The solution is only useful for testing. In separations, a current of the gas must always be used. It is best prepared in an apparatus like that shown in fig. 32, by acting on ferrous sulphide with dilute hydrochloric acid. When iron has to be subsequently determined in the assay solution, the gas should be washed by bubbling it through water in the smaller bottle ; but for most purposes washing can be dispensed with. The gas is very objectionable, and operations with it must be carried out in a cupboard with a good draught. When the precipitation has been completed, the apparatus should always be washed out. The effect of this acid on solutions of the metals is to form sulphides. All the metallic sulphides are insoluble in water; but some are soluble in alkaline, and some in acid, solutions. If sulphuretted hydrogen is passed through an acid solution containing the metals till no further precipitation takes place, a precipitate will be formed containing sulphides insoluble in the acid. On filtering, adding ammonia (to render the filtrate alkaline), and again passing the gas, a further precipitate will be obtained, consisting of sulphides insoluble in an alkaline solution, but not precipitable in an acid one; the filtrate may also contain sulphides not precipitable in an acid solution, which are soluble in an alkaline one ; these will be thrown down on neutralising. Again, the metals precipitated in the acid solution form sulphides which may be divided into groups, the one consisting of those which are soluble, and the other of those which are not soluble, in alkalies. This classification is shown in the following summary :—
1. Precipitable in an acid solution.
(а) Soluble in Alkalies.—Sulphides of As, Sb, Sn, Au, Pt, Ir, Mo, Te, and Se.
(b) Insoluble in Alkalies.—Sulphides of Ag, Pb, Hg, Bi, Cu, Cd, Pd, Rh, Os, and Ru.
2. Not precipitated in an acid solution, but thrown down in an alkaline one.
Sulphides of Mn, Zn, Fe, Ni, Co, In, Tl, and Ga.
These can again be divided into those which are dissolved by dilute acids and those which are not.
3. Not precipitated in an acid or alkaline solution, but thrown down on neutralising the latter.
Sulphides of V and W.
Sulphuretted hydrogen is a strong reducing agent. Ferric salts are thereby quickly reduced to ferrous; in hot solutions nitric acid is decomposed. These changes are marked by a precipitation of sulphur, and the student must be careful to pass the gas sufficiently long, and not be too hasty in concluding that no sulphide will form because it does not at once make its appearance. The best indication that it has been passed long enough is the smell of the gas in the solution after shaking.
Sulphurous Acid, H2SO3.—The reagent used may be regarded as a saturated solution of sulphur dioxide in water. It may be purchased, and keeps for a long time. It may be made by heating copper with sulphuric acid and passing the gas formed into water. The heat should be withdrawn when the gas is coming off freely. It is used as a reducing agent, and should not be diluted.
Sulphuric Acid, H2SO4. (Sp. gr. 1.84, containing 96 per cent, of real acid, H2SO4.)—This acid forms insoluble sulphates with salts of lead, strontium, and barium. It has a high boiling point, 290° C., and, when evaporated with salts of the more volatile acids, converts them into sulphates. When nitrates or chlorides are objectionable in a solution, evaporation with sulphuric acid removes them. In working with this acid caution is necessary, since, on mixing with water, great heat is evolved; and, if either the acid or water has been previously heated, a serious accident may result. In diluting the acid it should be poured into cold water. Glass vessels containing boiling sulphuric acid should be handled as little as possible, and should not be cooled under the tap. The action of diluted sulphuric acid on metals closely resembles that of dilute hydrochloric acid. Magnesium, aluminium, iron, zinc, nickel, cobalt, manganese, and cadmium dissolve, with evolution of hydrogen, in the cold acid, or when warmed. The action of hot and strong sulphuric acid is altogether different; it acts as an oxidising agent, and is itself reduced to sulphur dioxide or even to sulphur. The following metals are attacked in this way:—copper, bismuth, mercury, silver, antimony, tin, and lead. Gold, platinum, and arsenic are- not affected. This property is made use of in parting silver from gold and platinum. Metallic sulphides are similarly attacked; but this method of opening up minerals has the disadvantage of giving rise to the formation of anhydrous sulphates of iron, &c., which are not readily dissolved when afterwards diluted. The use of sulphuric acid in assaying is (for these reasons) to be avoided. Its chief use is as a drying agent, since it has a strong affinity for water. Air under a bell jar may be kept dry by means of a basin of sulphuric acid, and gases bubbled through it are freed from water-vapour.
Dilute Sulphuric Acid.—This is made by diluting 1 volume of the strong acid with 4 of water.
Tartaric Acid, H2T or C4H6O6.—A crystallised organic acid, soluble in less than its own weight of water, or in less than three parts of alcohol. It is used for the same purposes as citric acid is. The solution is made when required.
BASES, SALTS, &c.
Alcohol, C2H6O. (Commercial alcohol of sp. gr. 0.838 ; it contains 84 per cent, by weight of alcohol.)—It should burn with a non-luminous flame and leave no residue. It is used for washing precipitates where water is inapplicable, and for facilitating drying.
Ammonia, NH3. (Commercial ammonia, a solution having a sp. gr. of 0.88 to 0.89, and containing about 33 per cent, of ammonia.)—It is used as an alkali (more commonly than soda or potash), since an excess of it is easily removed by boiling. The salts of ammonium formed by it may be removed by igniting, or by evaporating in a porcelain dish with an excess of nitric acid. It differs in a marked way from soda or potash in its solvent action on the oxides or hydrates of the metals. Salts of the following metals are soluble in an ammoniacal solution in the presence of ammonic chloride:—copper, cadmium, silver, nickel, cobalt, manganese, zinc, magnesium, sodium, potassium, and the alkaline earths.
Dilute Ammonia is made by diluting 1 vol. of commercial ammonia with 2 of water. The dilute ammonia is always used; but in assays for copper a stronger solution (1 of strong ammonia to 1 of water) is required.
Ammonic Carbonate (Am3CO3) is prepared by dissolving one part of the commercial sesquicarbonate of ammonia in four parts of water, and adding one part of strong ammonia.
Ammonic Bicarbonate (HAmCO3) is prepared by saturating a solution of the sesquicarbonate of ammonia with carbon dioxide.
Ammonic Chloride, AmCl.—Use the commercial salt in a 20 per cent, solution in water. The salt should leave no residue on ignition.
Ammonic Molybdate.—The solution is prepared as follows : —Dissolve 100 grams of the powdered commercial salt in 200 c.c. of dilute ammonia, and pour the solution in a slow stream into 750 c.c. of dilute nitric acid; make up to 1 litre, and allow the mixture to settle before using. It is used for the purpose of separating phosphoric oxide from bases and from other acids, and also as a test for phosphates and arsenates. In using this solution the substance must be dissolved in nitric acid, and a considerable excess of the reagent added (50 c.c. is sufficient to precipitate 0.1 gram P2O5); when the phosphate is in excess no precipitate will be got. The precipitate is phospho-molybdate of ammonia.
Ammonic Nitrate (AmNO3) is used in the separation of phosphoric oxide by the molybdate method, and occasionally for destroying organic matter. It is soluble in less than its own weight of water. The solution is made when wanted.
Ammonic Oxalate (Am2C2O4.2H2O) is used chiefly for the separation of lime. The solution is made by dissolving 15 grams of the salt in 100 c.c. of water.
Ammonic Sulphide may be purchased in the state of a strong solution. It is yellow, and contains the disulphide, S2Am2. It serves the same purpose as is obtained by passing a current of sulphuretted hydrogen through an ammoniacal solution; but has the disadvantage of loading the solution with sulphur, which is precipitated when the solution is subsequently acidified. It is useful for dissolving the lower sulphide of tin (SnS).
Baric Carbonate (BaCO3) is sometimes used for precipitating the weaker bases. It should be prepared when wanted by precipitating a solution of baric chloride with ammonic carbonate and washing. The moist precipitate is used without drying.
Baric Chloride, BaCl2.2H2O.—A crystallised salt, soluble in 2½ parts of water. It is used for the detection and separation of sulphates. Make a 10 per cent, solution.
“Black Flux.”—A mixture of finely divided carbon with carbonate of potash or with carbonates of potash and soda. It is prepared by heating tartar or “rochelle salt” until no more combustible gas is given off. One gram will reduce about 2 grams of lead from litharge.
Borax, Na2B4O7.10H2O.—It is chiefly used as a flux in dry assaying, as already described. It is also used in testing before the blowpipe; many metallic oxides impart a characteristic colour to a bead of borax in which they have been fused.
Calcium Chloride.—The crystallised salt is CaCl2.6H2O ; dried at 200° C. it becomes CaCl2.2H2O, and when fused it becomes dehydrated. The fused salt, broken into small lumps, is used for drying gases. It combines with water, giving off much heat; and dissolves in a little more than its own weight of water. Strong solutions may be used in baths in which temperatures above the boiling-point of water are required. One part of the salt and 2 of water give a solution boiling at 112°, and a solution of 2 parts of the salt in 1 of water boils at 158°. The salt is very little used as a reagent.
Calcium Fluoride or “Fluor Spar,” CaF2.—The mineral is used as a flux in dry assaying; it renders slags which are thick from the presence of phosphates, &c., very fluid. Mixed with hydrochloric acid it may sometimes be used instead of hydrofluoric acid.
Calcium Carbonate, CaCO3.—It is precipitated in a pure state by ammonic carbonate from a solution of calcium chloride. It is used for standardising. In the impure state, as marble or limestone, it is used in the preparation of carbonic acid.
Calcium Hydrate or “Lime Water.”—This is used in testing for carbon dioxide and in estimating the amount of that gas present in air. It may be made by slaking quicklime and digesting the slaked lime with water. One hundred c.c. of water at 15° C. dissolves 0.1368 grams of the hydrate (CaH2O2), and hot water dissolves still less. “Milk of lime” is slaked lime suspended in water.
Cobalt Nitrate (Co(NO3)2.6H2O) is used in a 10 per cent, solution for the detection of oxides of zinc, aluminium, &c.; on ignition with which it forms characteristically coloured compounds.
Copper, Cu.—Pure copper, as obtained by electrolysis, can be purchased. This only should be used.
Copper Oxide, CuO,—It occurs as a black, heavy, and gritty power, and is used for the oxidation of carbon and hydrogen in organic substances. It should be ignited and cooled out of contact with air just before using, since it is hygroscopic. Oxide of copper which has been used may be again utilised after calcination.
Copper Sulphate (CuSO4.5H2O) contains 25.4 per cent, of copper. It is used in the outer cell of a Daniell-battery. The commercial salt is used for this purpose. The re-crystallised and pure salt is used for preparing the anhydrous sulphate, which is used for detecting moisture in gases. For this purpose it is dried at 200° C. till no trace of green or blue colour remains. It must be prepared when wanted. It may be conveniently used in the form of pumice-stone, saturated with a solution of the salt and dried. Traces of moisture develop a green colour.
Ferric Chloride, Fe2Cl6. (When crystallised, Fe2Cl6.6H2O.) —The solution is prepared as described under iron. The commercial salt contains arsenic, and, since the chief use of ferric chloride is for the determination of this substance, it must be purified (see under Arsenic).
Ferric Sulphate (Fe2(SO4)3) is a yellowish white deliquescent salt. It is used as an indicator in volumetric silver assaying, and for the separation of iodine from bromine. It may be purchased as iron alum, Am2Fe2(SO4)4.24H2O. But it is best prepared by adding strong sulphuric acid to ferric hydrate in equivalent proportions. Use it as a solution containing 2 or 3 per cent, of iron.
Ferrous Sulphate, FeSO4.7H2O.—The granulated form is best, and can be purchased pure. It is used for standardising. It keeps better in crystals than in solution. It is readily soluble in water, but the solution is best made with the help of a little free acid. As a re-agent use a 10 per cent, solution. The crystals should be clear bluish-green ; if their colour is dark green, brown, or blue, they should be rejected.
Ferrous Sulphide (FeS) is used for the preparation of sulphuretted hydrogen. It may be purchased and broken in small lumps, nut-size, for use.
“Fusion Mixture” (K2CO3.Na2CO3) is a mixture of potassic and sodic carbonates in the proportions of 13 of the former to 10 of the latter, by weight. It is hygroscopic. A mixture of the bicarbonates is better, being purer and less apt to get damp.
Gallic Acid (C7H6O5.H3O) is an organic acid, occurring as a pale fawn-coloured crystalline powder, soluble in 100 parts of cold water, or in 3 parts of boiling water. It is used for the determination of antimony. A 10 per cent, solution in warm water is made when required.
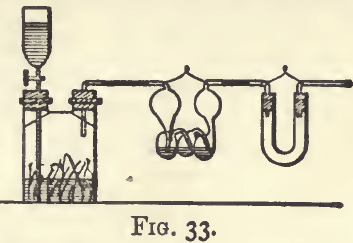
Hydrogen (H) is a gas. It is obtained by acting on zinc with dilute hydrochloric or sulphuric acid. It is used as a reducing agent, and for providing an atmosphere free from oxygen. It reduces metallic oxides at a high temperature. It must be freed from water; and special precautions should be taken to prevent an admixture with air. It is generally required in a current which can be continued for an hour or more without interruption. The preparation can be conveniently carried out in the apparatus shown (fig. 33). A quart bottle is half filled with sheet zinc, and connected with bulbs filled with sulphuric acid, and with a calcium chloride tube. The last is connected with the apparatus through which the gas has to be passed. Dilute hydrochloric acid mixed with a few cubic centimetres (20 c.c. to 1 pint) of stannous chloride sol. to fix any dissolved oxygen, is placed in the funnel, and let into the bottle by opening the stopcock when required. Care must be taken to let the hydrogen escape for some time before starting the reduction.
Gold, Au.—Gold, obtained by cupelling and “parting,” is for most purposes sufficiently pure. It is best kept in the shape of foil. When the purer metal is required, gold should be dissolved in aqua regia, the solution evaporated to a paste, diluted, allowed to stand, and filtered. The filtered solution is acidified with hydrochloric acid, warmed, and precipitated with sodium sulphite. The precipitate is collected, washed, and fused on charcoal.
Iron, Fe.—The soft wire (thin) is used for standardising. Rods are used in dry assays as a desulphurising agent. Steel must not be used, since it is not pure, and contains a variable amount of iron.
Lead, Pb.—Granulated lead or lead-foil is used in the dry assay for silver and gold, and in the preparation of lead salts. It can be obtained very pure, but always contains more or less silver, 1 or 2 milligrams in 100 grams. The amount of silver it contains must be determined and recorded.
Lead Acetate (PbAc2.3H2O, or Pb(C2H3O2)2.3H2O) is used as a test, specially for the detection and estimation of sulphuretted hydrogen. Prepare a 10 per cent, solution for use.
Lead Nitrate (Pb(NO3)5) can be purchased pure. It is used for standardising.
Lead Dioxide (PbO2) occurs as a dark-brown powder. It is used as an oxidizing agent and for absorbing sulphurous oxide. It can be prepared by digesting red lead with warm dilute nitric acid ; washing and drying the residue.
“Litharge,” PbO.—It can be purchased as a yellow heavy powder. It is used in dry assaying as a flux, as a desulphurising , agent, and also as a source of lead. It always contains some silver, the amount of which must be determined.
Litmus.—This is an organic colouring matter which is turned red by acids and blue by alkalies. For ordinary purposes it is best used as litmus paper, which may be purchased in small books. A solution is prepared by digesting 15 or 20 grams of the commercial litmus in 100 c.c. of water on the water bath. After being allowed to settle, it is filtered and made just faintly red with acetic acid. Then there is added a drop or two of a solution of soda and 10 c.c. of alcohol. It should be kept in a loosely-covered bottle.
Magnesia, MgO.—It maybe purchased as “calcined magnesia.” It is used for making “magnesia mixture,” and should be kept in a corked wide-mouthed bottle.
“Magnesia Mixture.”—Dissolve 22 grams of magnesia in about a quarter of a litre of dilute hydrochloric acid, avoiding excess. Add 5 grams of magnesia, boil, and filter. Add 300 grams of ammonic chloride, and 250 c.c. of strong ammonia; and dilute with water to 2 litres. It should be kept in a stoppered winchester.
Magnesium Sulphate, MgSO4.7H2O.—It can be purchased very pure, and is occasionally used as a standard salt.
Manganese Dioxide, MnO3.—It is used in the preparation of chlorine. The commercial article is not pure, but is sufficiently so for this purpose.
Marble, CaCO3.—Fragments of the white crystalline variety only should be used. It is used as a source of lime and of carbon dioxide.
Mercury, Hg.—This can be purchased pure. It should have a bright surface, flow without a tail, and leave no residue on ignition. It is used as a standard ; for amalgamation ; and as a confining liquid in gas analysis.
Mercuric Chloride (HgCl2) may be purchased pure. Make a 5 per cent, solution in water. It is used for destroying an excess of stannous chloride ; for removing sulphuretted hydrogen from solution; and as a test for stannous salts.
Microcosmic Salt, HAmNaPO4.8H2O.—When fused NaPO3 is formed. It is used in testing for metallic oxides and silica before the blowpipe. The crystals are sometimes used as a standard for phosphoric acid.
“Nessler’s Solution.”—Mode of preparation : Dissolve 35 grams of potassium iodide in 100 c.c. of water; dissolve 17 grams of mercuric chloride in 300 c.c. of water, and pour this solution into that of the iodide till a permanent precipitate is produced ; make up to 1 litre with a 20 per cent, solution of potash; add mercuric chloride till a precipitate is again formed; allow to settle and decant. It is used for detecting ammonia.
Nitre.—This is potassium nitrate.
Platinum Chloride, 2HCl.PtCl4. (In the crystallised form it has 6H2O).—It may be made as follows :—Take 5 grams of clean platinum scrap and dissolve in a flask at a gentle heat in 50 c.c. of hydrochloric acid with the occasional addition of some nitric acid; evaporate to a paste; and then dissolve in 100 c.c. of water. It is used for separating and determining potassium.
Phenolphthalein is an organic compound used as an indicator; more especially in determining the weaker acids. It cannot be used in the presence of ammonia. Dissolve half a gram in 100 c.c. of dilute alcohol.
Potassium Bicarbonate, KHCO3.—It may be purchased pure; on ignition it leaves the carbonate, K2CO3, which may be used as a standard.
Potassium Cyanide, KCN.—It is used in the dry assay as a reducing agent. The commercial salt is very impure. Purchase that sold as potassic cyanide (gold) which contains about 95 per cent, of KCN. It is used for copper assaying and occasionally in separation. Make a 10 per cent, solution when wanted.
Potassium Bichromate, K2Cr2O7. It may be purchased nearly pure. It is used as an oxidising agent, for determining iron; and as a test solution. For this last purpose a 10 per cent, solution is prepared.
Potassium Chlorate (KClO3) can be purchased pure. It is used with hydrochloric acid as a substitute for aqua regia.
Potassium Ferrocyanide (K4Fe(CN)6.3H2O), or “yellow prussiate of potash,” is used as a test; as an indicator; and for the determination of zinc. Make a 5 per cent, solution.
Potassium Ferricyanide (K6Fe2(CN)12), or “red prussiate of potash,” is used for testing ; and as an indicator. Make a 5 per cent, solution when wanted, as it decomposes on keeping.
Potassium Hydrate, KHO. Purchase that purified with alcohol. It is an alkali, and is used for absorbing carbonic acid, &c.
Potassium Iodide, KI. It may be purchased nearly pure. It is used as a test and for dissolving iodine. It should be used in a 10 per cent, solution freshly made. The solution decomposes on exposure to light, with separation of iodine.
Potassium Nitrate (KNO3) can be purchased pure. It is used in the dry way as an oxidizing agent. It is very fusible. It decomposes at a low temperature into potassium nitrite (KNO2) and free oxygen; and at a higher temperature leaves potash (K2O). It oxidizes sulphur and carbon with explosive violence. This action may be moderated by mixing the nitre with carbonate of soda, common salt, or some other inert body.
Potassium Nitrite, KNO2.—The commercial article is not pure, but is sufficiently so for the purpose required. A saturated solution is used in the separation of cobalt ; the solution is made when wanted.
Potassium Permanganate, KMnO4. This salt can be purchased sufficiently pure. It is much used as an oxidizing agent.
Potassium Bisulphate (KHSO4) is used as a dry reagent for opening up minerals. It fuses ; and at a much higher temperature is converted into potassium sulphate with loss of sulphuric acid.
Potassium Sulphocyanate (KCNS) is used for the detection and determination of traces of ferric iron; as also in the separation of silver and copper from some of the other metals. Make a 10 per cent, solution. It should show no colour on the addition of hydrochloric acid.
“Red Lead” (Pb3O4) is used in the dry assay as a flux instead of litharge, from which it differs in containing a little more oxygen. When acted on by nitric acid a brown residue of lead dioxide is left, nitrate of lead going into solution. Like litharge it always carries silver; about 2 milligrams in 100 grams.
Silver, Ag.—Pure silver in foil is required as a standard. It may be prepared as follows:—Dissolve scrap silver in dilute nitric acid and decant off from any residue ; dilute the solution with hot water and add hydrochloric acid until there is no further precipitate, stir ; allow the precipitate to settle ; decant and wash ; dry the precipitate, mix it with twice its bulk of carbonate of soda and fuse the mixture in a crucible until tranquil; clean the button and roll or hammer it into foil.
Sodium Acetate, NaC2H3O2.3H2O.—The crystals may be purchased sufficiently pure. Make a 20 per cent, solution in water. It is used for replacing mineral acids by acetic acid.
Sodium Acetate and Acetic Acid.—A solution is used in the determination of phosphates and arsenates; 100 grams of the salt is dissolved in 500 c.c. of acetic acid, and diluted with water to one litre.
Sodium Bicarbonate (NaHCO3)is used as a flux in dry methods. On ignition it leaves the carbonate (Na2CO3), which is used as a standard reagent. Make a 20 per cent, solution of the carbonate for use. It should be free from chlorides or sulphates, or if impure the amount of impurities must be determined.
Sodium Hydrate, NaHO. It may be purchased in sticks, which should be kept in a well-corked bottle. It is sometimes called “caustic soda.” It is a strong alkali. It is used for neutralizing acid solutions and for separations where ammonia is unsuitable. Make a 5 per cent, solution for use.
Sodium Hyposulphite, Na2S2O3.5H2O.—It may be purchased pure. It is generally known as “ hypo.” It is used as a standard.
Sodium Sulphite (Na2SO3.7H2O) is used as a reducing agent.
Sodium Phosphate, Na2HPO4.12H2O. The crystals may be purchased pure, but they effloresce in dry air with loss of water.
It is used as a standard and for precipitating magnesia, &c. Make a 10 per cent, solution.
Stannous Chloride, SnCl2.2H2O.—The crystals are best purchased. If kept dry and free from air they are fairly permanent. A solution is made by dissolving 20 grams in 10 c.c. of hydrochloric acid and diluting to 1 litre. The solution is not permanent. It is a strong reducing agent, and is chiefly used in solution for this purpose.
Tin, Sn.—Grain tin should be purchased. It is not pure, but contains 99.5 per cent, of the metal. The chief impurity is copper. It can be used as a standard. When acted on with hot hydrochloric acid it slowly dissolves (more rapidly in contact with platinum) and forms stannous chloride.
Uranium Acetate, UO2(C2H3O2)2.H2O.—It is best purchased in crystals. The solution is used for the determination of phosphates and arsenates. A solution of 3 per cent, strength is occasionally used as an indicator.
Uranium Nitrate, UO2(NO3)2.6H2O.—This salt is very soluble in water and is sometimes used instead of the acetate, which is somewhat difficult to dissolve.
“Water,” H2O.—Spring or well water is sufficiently pure for most purposes, 100 c.c. will leave a residue of from 10 to 30 milligrams, so that where a salt has to be dissolved out, evaporated, and weighed it should be replaced by distilled water. Rain water, melted snow, &c., always leave less residue than spring water; but in other respects they are often dirtier. Distilled water is best prepared in the office, a glass or tin condenser being used.
Zinc, Zn.—It is sold in a granulated form or in sticks. It generally contains over 1 per cent, of lead, with a little iron and arsenic. It is used for separating metals from their solutions, and generally as a reducing agent. For the preparation of hydrogen, and in most other cases, scrap sheet zinc may be used.
Zinc Oxide, ZnO.—The commercial oxide sometimes contains carbonate. .
Zinc Sulphate, ZnSO4.7H2O.—It is occasionally used as a standard, and can be purchased nearly pure.
