Weighing.—The system of weights and measures which we have adopted is the French or metric system; in this the gram (15-43 grains) is the unit of weight; the only other weight frequently referred to is the milligram, which is 0.001, or 1/1000 gram. The unit of volume is the cubic centimetre, which is approximately the volume of 1 gram of water, and which thus bears to the gram the same relation as grain-measures bear to grains. It is usual to write and even pronounce cubic centimetre shortly as c.c., and the only other denomination of volume we shall have occasion to use is the “ litre,” which measures 1ooo c.c., and is roughly 1¾ pints.
The weights used are kept in boxes in a definite order, so that the weights on the balance can be counted as well by noting those which are absent from the box as by counting those present on the scale-pan. The weights run 50, 20, 10, 10, 5, 2, 1, 1 and 1 grams, and are formed of brass. The fractions of the gram are generally made of platinum or of aluminium, and are arranged in the following order :—0.5, 0.2, 0.1, 0.1, and 0.05, 0.02, 0.01, 0.01. These may be marked in this way, or they may be marked 500, 200, 100, 100, 50, 20, 10, 10 ; the 500 meaning 500 milligrams.
Some makers send out weights in the series 50, 20, 20, 10, &o.
Weights of less than 0.01 gram are generally present in a box, but it is much more convenient to work with a rider. This is a piece of wire which in the pan weighs 0.01 gram; it is made in such a form that it will ride on the beam, and its effective weight decreases as it approaches the centre. If the arm of the beam is divided into tenths, then each tenth counting from the centre outward equals 0.001 gram or 1 milligram, and if these tenths be further subdivided the fractions of a milligram are obtained; and these give figures in the fourth place of decimals. A fairly good balance should be sensitive to 0.0001 gram. The weights must never be touched with the fingers, and the forceps for moving them is used for no other purpose. When not in actual use the box is kept closed. The weights must not be allowed to remain on the pan of the balance. The balance-case must not be open without some reason. It must be fixed level, and, once fixed, must not be needlessly moved. The bench on which it stands should be used for no other purpose, and no one should be allowed to lean upon it.
When using a balance sit directly in front of it. Ordinarily the substance to be weighed is best put on the pan to the user’s left; the weights and the rider are then easily manipulated.
Powders should not be weighed directly on the balance; a counterpoised watch-glass or metal scoop (fig. 25) should be used. In some cases it is advisable to use a weighing-bottle. This is a light, well-stoppered bottle (fig. 3) containing the powdered ore. It is first filled and weighed ; then some of the substance is carefully poured from it into a beaker or other vessel, and it is weighed again; the difference in the two weighings gives the weight of substance taken. A substance must always be cold when weighed, and large glass vessels should be allowed to stand in the balance-box a little while before being weighed. Always have the balance at rest when putting on or taking off anything from the pans. Put the weights on systematically. In using the rider (except you have a reason to the contrary), put it on at the 5; if this is too much, then try it at the 3 ; if then the weights are too little, try at the 4, if still not enough, the correct weight must be between the 4 and 5 ; try half-way between.

It is best to work with the balance vibrating; equilibrium is established when the vibration to the left is the mean of the preceding and succeeding vibrations to the right. For example, if it vibrates 6 divisions to the right on one swing, and 5 divisions on the next, the intermediate vibration to the left should have been 5½.
Note whether the substance increases in weight whilst on the balance. If it does it may be because it was put on warm, and is cooling, or it may be because it is taking up moisture from the air. Substances which take up moisture rapidly should be weighed in clipped watch-glasses or in light-weighing bottles or tubes.
Students, in recording the weights, should first read off those missing from the box, writing down each order of figures as determined; first tens, then units, and so on. Remember that the first four platinum weights give the figures of the first place of decimals, the second four give the second place, and that the third and fourth places are given by the rider. Having taken down the figures, confirm them by reading off the weights as you put them back into the box. Do not rest a weight on the palm of your hand for convenience in reading the mark upon it. Remember one weight lost from a box spoils the set. Do not take it for granted that the balance is in equilibrium before you start weighing: try it.
Measuring Liquids.—For coarse work, such as measuring acids for dissolving ores, graduated glasses similar to those used by druggists may be used. It is well to have two sizes—a smaller graduated into
divisions of 5 c.c. (fig. 26), and a larger with divisions equal to 10 c.c. No measurement of importance should be made in a vessel of this kind, as a slight variation in level causes a serious error.

Graduated flasks must be used when anything has to be made up to a definite bulk, or when a fixed volume has to be collected. If, for example, a certain weight of substance has to be dissolved and diluted to a litre, or if the first 50 c.c. of a distillate has to be collected, a flask should be used. Each flask is graduated for one particular quantity; the most useful sizes are 1000 c.c., 500 c.c., 200 c.c., 100 c.c., and 50 c.c. The mark should be in the narrowest part of the neck, and should be tangential to the curved surface of the liquid when the flask contains the exact volume specified. The level of a curved surface of liquid is at first somewhat difficult to read : the beginner is in doubt whether the surface should be taken at A, B, or C (fig. 27). It is best to take the lowest reading c. In some lights it is difficult to find this; in such cases a piece of white paper or card held behind and a little below, so as to throw light up and against the curved surface, will render it clear. In reading, one should look neither up at nor down upon the surface, but the eye should be on the same level with it. It must be kept in mind that flasks contain the quantity specified, but deliver less than this by the amount remaining in them and damping the sides.
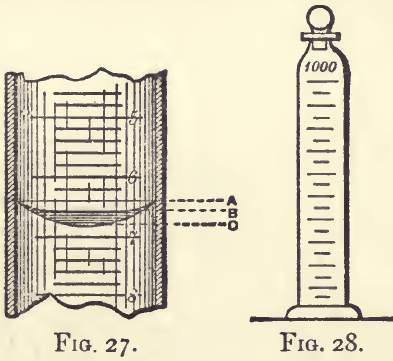
If it is desired to transfer the contents say of a 100 c.c. flask to a beaker, it will be necessary to complete the transfer by rinsing out the flask and adding the washings ; otherwise there will be a sensible loss. Graduated cylinders (fig. 28) are convenient for preparing standard solutions.
Pipettes and burettes are graduated to deliver the quantities specified. The principle of the pipette, and the advantages and disadvantages of its various forms, may be understood by considering the first form shown in fig. 29. It is essentially a bulbed tube drawn out to a jet at its lower end, and having on each side of the bulb a mark so placed that when the surface of the liquid falls from the upper to the lower mark the instrument shall deliver exactly 100 c.c. The bore of the jet should be of such a size as will allow the level of the liquid to fall at the rate of about one foot in two minutes. If it runs more quickly than this, an appreciable error arises from the varying amount of liquid remaining, and damping the sides of the bulb. The flow of liquid from a pipette must not be hastened by blowing into it. The lower tube or nose of the pipette should be long enough to reach into the bottle or flask containing the liquid about to be measured. The pipette is filled by sucking at the open end with the mouth ; this method of filling renders the use of the instrument dangerous for such liquids as strong acids, ammonia, and such poisonous solutions as that of potassic cyanide. One attempt with a fairly strong solution of ammonia will teach the beginner a very useful lesson. As soon as the liquid rises above the upper mark in the pipette, the mouth is withdrawn, and the pipette quickly closed by pressing the upper aperture with the index finger of the right hand; it is well to have the finger slightly moist, but not damp. The neck of the pipette should be long enough to allow its being firmly grasped by the fingers and thumb of the right hand without inconvenience.
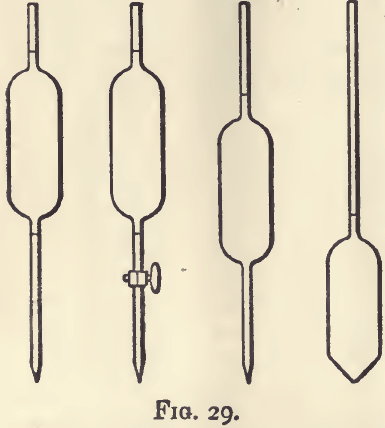
The pipette is first held in a vertical position long enough to allow any moisture outside the tube to run down, and then the liquid is allowed to run out to the level of the upper mark; this is easily effected by lessening the pressure. If the finger is wet, the flow will be jerky, and good work impossible. The pipette is next held over the vessel into which the 100 c.c. are to be put, and the liquid allowed to run out. When the bulb is nearly empty, the flow should be checked by replacing the finger, and the liquid allowed to escape slowly until the lower mark is reached. The pipette is then withdrawn; it is in the withdrawing that the disadvantage of this particular form makes itself felt. It must be withdrawn very steadily, as the slightest shock causes the remaining column of liquid to vibrate, whereby air is drawn in and the liquid is forced out.
This disadvantage is got rid of by making the mouth of the jet the lower limit, or, in other words, allowing the instrument to empty itself. There are two forms of such pipettes; in the one generally recommended in Gay-Lussac’s silver assay (the last shown in fig. 29) the nose is replaced by a jet. This is most conveniently filled by stopping the jet with the finger, and allowing the liquid to flow in a fine stream into the neck until the pipette is filled, and then working as just described. The other form is the one in general use; in fact, a long nose to a pipette is so convenient that it may almost be said to be necessary. But the accuracy is slightly diminished; a long narrow tube makes a poor measuring instrument because of the amount of liquid it finally retains. A defect possessed by both forms is the retention of a drop of varying size in the nozzle. Whatever method is adopted for removing this drop must be always adhered to. The most convenient form is the one last described, and the most useful sizes are 100 c.c., 50 c.c., 20 c.c., 10 c.c., and 5 c.c. Ten c.c. pipettes graduated into tenths of a cubic centimetre are very useful: those are best in which the graduation stops short of the bottom.
All measurements should be made at the ordinary temperature; and, before being used, the pipette should be rinsed out with a cubic centimetre or so of the solution to be measured. After using, it should be washed out with water.
Burettes differ mainly from pipettes in having the flow of liquid controlled from below instead of from above. The best form is that known as Mohr’s, one kind of which is provided with a glass stopcock, while the other has a piece of india-rubber tube compressed by a clip. The latter cannot be used for solutions of permanganate of potash or of iodine, or of any substance which acts on india-rubber ; but in other respects there is little to choose between the two kinds. A burette delivering 100 c.c., and graduated into fifths (i.e., each division = 0.2 c.c.), is a very convenient size. For some kinds of work, 50 c.c. divided into tenths (i.e., each division = 0.1 c.c.) may be selected.
Burettes may be fixed in any convenient stand; they must be vertical and should be so placed that the assayer can read any part of the graduated scale without straining. When not in use, they should be kept full of water. When using a burette, the water must be run out; the burette is next rinsed with some of the solution to be used, and drained ; and then it is filled with the solution. Next squeeze the india-rubber tube so as to disentangle air-bubbles and, by smartly opening the clip, allow the tube and jet to be filled; see that no bubbles of air are left. Then run out cautiously until the level of the liquid in the burette stands at zero. In reading the level with very dark-coloured liquids it is convenient to read from the level A (fig. 27), and, provided it is done in each reading, there is no objection to this. The accuracy of the reading of a burette is sensibly increased by the use of an Erdmann float. This is an elongated bulb, weighted with mercury, and fitting (somewhat loosely) the tube of the burette. It floats in the solution,
and is marked with a horizontal line; this line is taken as the level of the liquid. If the burette is filled from the top, the float rises with aggravating slowness, and this is its chief disadvantage. The float must come to rest before any reading is made.
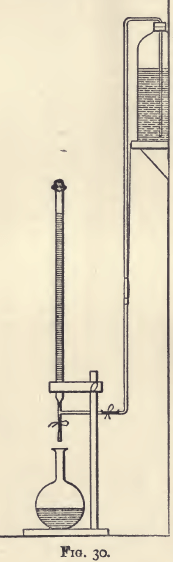
A convenient plan for filling a burette from below is shown in fig. 30. The diagram explains itself. The bottle containing the standard solution is connected with the burette by a syphon arrangement through the glass tube and T-piece. The flow of liquid into the burette is controlled by the clip. When this clip is opened, the burette fills; and when it is closed, the burette is ready for use in the ordinary way.
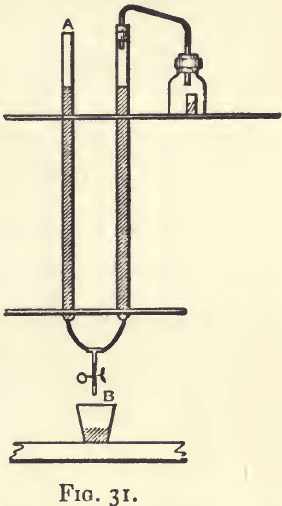
Measuring Gases.—Lunge’s nitrometer (fig. 69) is a very convenient instrument for many gasometric methods. It requires the use of a fair quantity of mercury. In fig. 31, there is a representation of a piece of apparatus easily fitted up from the ordinary material of a laboratory. It is one which will serve some useful purposes. It consists of a wide-mouthed bottle fitted (by preference) with a rubber cork. The cork is perforated, and in the perforation is placed a glass tube which communicates with the burette. The burette is connected by a rubber tube and a Y-piece, either with another burette or with a piece of ordinary combustion-tube of about the same size. The wide-mouthed bottle contains either a short test-tube or an ordinary phial with its neck cut off. In working the apparatus the weighed substance is put in the bottle and the re-agent which is to act on it, in the test-tube; the cork is then inserted. The liquid in the two burettes is next brought to the same level, either by pouring it in at A or running it out at B. The level of the liquid in the apparatus for correcting variation in volume is then read and noted. Next, after seeing that the level of the liquid in the burette has not changed, turn the bottle over on its side so that the re-agent in the test-tube shall be upset into the bottle. Then, as the volume of the gas increases, lower the liquid in the burette by running it out at B, and at the same time keep the level in a half an inch or so lower than that in the burette. When the action has finished bring the liquid in the two vessels to the same level and read off the burette. This part of the work must always be done in the same manner.
The volume corrector for gas analysis is a graduated glass tube of 120 c.c. capacity inverted over a narrow glass cylinder of mercury. It contains 0.2 or 0.3 c.c. of water and a volume of air, which, if dry and under standard conditions, would measure 100 c.c. The actual volume varies from day to day, and is read off at any time by bringing the mercury inside and outside to the same level. This is done by raising or lowering the tube, as may be required. Any volume of gas obtained in an assay can be corrected to standard temperature and pressure by multiplying by 100 and dividing by the number of c.c. in the corrector at the time the assay is made.
