Table of Contents
For technical purposes the following estimations may be required:
- Moisture, Volatile Matter, Ash, and Fixed Carbon; Sulphur, and Phosphorus.
- Carbon and Hydrogen by ultimate organic analysis.
- The calorific (or heating) power of the coal.
Besides the chemical analysis of cokes, there are required the “Crushing resistance,” “Porosity,” and “Specific Gravity.” For information concerning these tests, and the more accurate methods of estimating the calorific power, consult Stillman’s Engineering Chemistry and Poole’s Calorific Power of Fuels. In the last mentioned work especially, and in Dana’s System, of Mineralogy, the student will find very complete lists of analyses of the many different kinds of coals. The student must remember, when considering the merits of a coal, that besides the chemical, calorific, and other tests, he must consider the price of the coal. A coal inferior somewhat on these tests to a good steam coal may be recommended, if otherwise favourable, in preference to the good coal, provided the difference of prices on delivery more than counterbalances these deficiencies.
Analysis of Coal
Sample the coal, and obtain a 20 gm. sample which has passed through a 100 mesh sieve. From this, carefully sample a few grams, and further reduce in the agate mortar.
(a) Moisture.—Weigh out two gms. of this sample. Transfer to a weighed 25 c.c. platinum crucible. Place in the air bath, regulated at 105° C., for thirty minutes. Cool in a desiccator and weigh. The loss in weight represents moisture. On further heating it will be found that this loss increases, but any pyrites present is apt to oxidise; therefore it is preferable, when examining coals, to treat all alike for thirty minutes, and consider the result as moisture. The result, though probably inaccurate, is yet sufficient in that it gives a relative idea of the value of various coals.
(b) Volatile Matter (mostly combustible).—The crucible with lid and contents from (a) are now placed over a good single bunsen and cautiously heated to redness for three minutes, then over the blast (gas blowpipe) for other three minutes. Cool in a desiccator and weigh. The loss caused by this ignition is the volatile (mostly combustible) matter, plus one-half of the total sulphur present.
(c) Fixed Carbon. -The crucible and contents from (b) are now placed over the bunsen, the lid being removed. Gentle ignition is continued (assisted by a slow current of oxygen if combustion be very slow) until it is seen that all carbonaceous particles have been consumed. The loss in weight caused by this ignition represents the fixed carbon.
(d) Ash.—The residue left in the crucible is the ash. The difference in weight between ‘ crucible + lid ’ and crucible + lid + residue = ash.
Note.—The student must rigidly adhere to the times of ignition given above. The results so obtained, though only relative, serve for the comparison of various solid fuels as regards these constituents.
(e) Sulphur.—Finely powder in the agate mortar another sample of about 2 gm. coal. From this weigh out 1 gm. Thoroughly mix in a wedgwood mortar with 8 gms. anhydrous Na2CO3 and 5 gms. KNO3. Transfer about one-tenth of the mixture to a large platinum crucible (40 c.c.). Heat to redness, and when combustion is complete add more of the mixture, and continue till all the charge is in the crucible. (If a small crucible is used, either use half the quantities or perform the operation in two stages.) Continue heating for a further fifteen minutes to make sure of completely oxidising the carbon. Cool and transfer to a 300 c.c. beaker, adding about 150 c.cs. water, and gently warm till the mass dissolves. Remove the crucible on a glass rod, washing it down with hot water, keeping it above the beaker. When the outside is washed, grasp the crucible in the fingers and wash out the inside. Filter and wash. Neutralise the solution with HCl. Add an excess of 2 c.cs. 16E. HCl. Boil, and add excess of BaCl2 solution. Let stand in a warm place for twelve hours. Filter, preferably through a “Gooch”; wash well; dry. ignite, and weigh as usual.
The weight of BaSO4 multiplied by 0.1374 gives the weight of S present in the ore taken, 1 gram. From this the percentage is easily calculated.
One-half of this amount has to be deducted from the volatile matter and one-half from the fixed carbon, as in the previous estimations the sulphur was included in these two items.
(f) Phosphorus.—As in the ash of the coal there may be less than 1 per cent, of phosphoric acid, it is evident that to obtain an accurate estimation at least .5 gm. ash must be taken for analysis. To obtain this quantity, finely grind 5 or 6 grams of the sample and weigh out 5 gms. Transfer to a wide shallow platinum basin (a piece of foil carefully turned up at the edges, and about 5 cms. square, will do). Place on a platinum triangle, and heat very carefully at a dull red till most of the volatile matter has disappeared. Raise the tempera¬ture and continue the combustion at a bright red till the carbonaceous matter has disappeared. The ash is now ready for analysis by any of the known methods.
To bring it into a soluble form, mix the residue with 1 gm. KNO3 and 4 gms. Na2CO3. Fuse till perfectly fluid in a platinum crucible. Dissolve the mass in 50 c.cs. dilute HCl (E.) in a deep beaker. Evaporate almost to dryness, adding towards the end of the evaporation 10 c.cs. HNO3 (16E.). When almost dry, take up with 6 c.cs. HNO3 (16E.) and 4 c.cs. H2O. Filter through a Gooch crucible. Wash well, bringing the bulk of liquid up to about 50 c.cs. Add 30 c.cs. of ammonium molybdate reagent. Let stand for three hours at 40° C., stirring now and then. Filter and wash with HNO3 (E.) till free from iron, then with water till free from acid. Check the filtrate to see if precipitation was complete. If a Gooch crucible was used, the precipitate may be dried at 100° C. and weighed. The weight thus found multiplied by .0373 gives the P2O5 present, or by .0179 gives the P present.
(g) The Calorific or Heating Power of Coal.—Of the many methods used in this determination, the following are a few:—
(1) Reduction of Litharge (easy, but inaccurate).
(2) By calculation from the Carbon and Hydrogen contents present (laborious, but where volatile matter is less than 20 per cent, fairly accurate).
(3) By combustion in a calorimeter such as a Thomson, Mahler, or other apparatus. Thomson’s gives approximate results relatively accurate. Mahler’s bomb is to be recommended for both accuracy
and simplicity.
Of these various methods the first two will be considered here. Mahler’s or Thomson’s methods will be found described in Poole’s Calorific Power of Fuels, or Stillman’s Engineering Chemistry.
(1) By Reduction of Litharge.
Finely powder a sample of the coal in an agate mortar. Weigh out 1 gm. of the powdered coal. Thoroughly mix with 30 gms. litharge by grinding in a wedgwood crucible. Transfer to an E crucible (Battersea round). Clean out the mortar with 20 gm. litharge, and add as a cover on top of the charge.
Fire at a bright red heat (using a lid) for 20 minutes. Remove, cool, break, collect and weigh the lead. Run a duplicate.
Theoretically, 2PbO + C = 2Pb + CO2 or 12 parts by weight of carbon, reduce 410 parts of lead, or 1 part by weight of carbon reduces 34 parts by weight of lead.
Assume that 1000 gms. pure carbon = 8140 calories (consult a text-book on Heat), then one part of lead represents 8140/34 = 239 calories.
Then if the button from the coal weigh 30.2 gms. the heating value of the coal is 239 x 30.2 = 7217.8 calories per ‘kilo’ (1000 gms,). In this manner the heating value of coals relative to that of pure carbon is approximately ascertained.
Note.—This is really a determination of the reducing power of the coal, not the heating power. The results are only approximate.
(2) By Calculation from the Carbon and Hydrogen contents, as determined by ultimate Organic Analysis.
This method will serve both as a check on the previous one and as an exercise in ultimate organic analysis. By ‘ ultimate ’ is meant the determination of the percentages of the several elements in contrast to ‘proximate’ analysis, in which the percentages of combinations of elements such as CH4 are estimated.
Before proceeding to the analysis the apparatus must be fitted up. Some care is here necessary, but a little time and care in thoroughly fitting and connecting each part will save subsequent vexatious delay.
Taken in order, the necessary apparatus is as follows:—Oxygen holder; apparatus for the purification of the oxygen; the combustion tube; the combustion furnace; the calcium chloride tube for the absorption of water and the potash bulbs for the absorption of carbon dioxide.
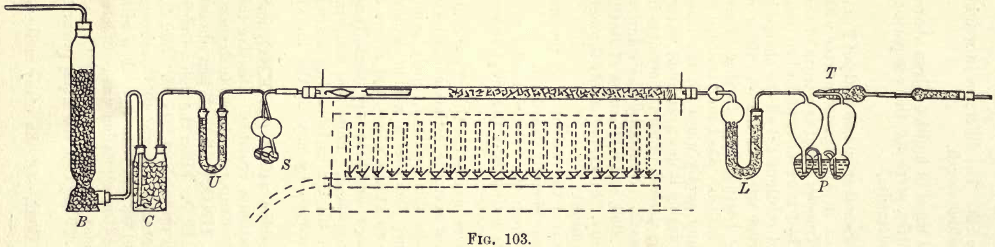
The arrangement of the apparatus is shown in fig. 103.
The oxygen may be stored in a cylinder, gas bag, or other suitable apparatus capable of delivering it under slight pressure.
The purification apparatus consists of a cylinder B filled with pumice moistened with strong H2SO4. This bottle is connected to the drying bottle C, filled with small lumps of solid KHO. Between this bottle
and the combustion tube are inserted a U tube (U) filled with soda lime and a set of “potash bulbs” (S) filled with strong H2SO4.
The combustion tube is a piece of hard Bohemian glass tubing, about 75 cm. long and 2 cm. diameter, open at both ends. This tube is filled and fitted as in fig. 104.

c, granular copper oxide; a, plug of asbestos ; p, a porcelain or platinum boat. The end connections are made with good, tight-fitting corks, screens, and tubing, as shown.
The combustion furnace consists of a row of bunsens, each with a tap. The top of the furnace is adapted to accommodate the tube.
The calcium chloride tube L is almost filled with dry granular calcium chloride, in pieces about the size of a small pea or grain of wheat. A small plug of cotton-wool is placed on top of the chloride, and then the tube is stoppered either by a sealed cork and leading tube or by ground glass stopper and tube. The tube is connected up as shown.
The “potash bulbs” may be the Liebig, as shown in the purification apparatus, or the Geissler as shown at P. The bulbs are charged about one-third full of a 30% solution of KHO, which is introduced by gentle suction. The projecting tube T is filled with small lumps of solid KHO.
When not in use, the ends of all these drying and absorption tubes are closed with small pieces of rubber tubing and short lengths of glass rod.
Since in the analysis carbon and hydrogen are oxidised to carbon dioxide and water, it is necessary before commencing the analysis to completely remove from the combustion tube all traces of such substances. To do this, disconnect the chloride tubes and potash bulbs. Gently heat the glass tube along its entire length; bring the temperature up to a low red. Continue at this temperature for 20 minutes, keeping a slow stream of oxygen passing through the tube.
Weigh the CaCl2 tube and KHO bulbs, removing the rubber caps before weighing. This weighing can be done whilst the former operation is proceeding.
Connect the tubes and bulbs, and continue passing oxygen at the rate of about two bubbles per second for 15 minutes. Disconnect the tubes and bulbs, and reweigh. If there is a gain of more than 2 or 3 milligrams, continue the operation till constant within this limit, taking care that moisture does not condense on the outside of the glass in cold weather. Test the whole apparatus for leaks, by connecting a small tube bent at right angles to the inlet of the drying cylinder on the left. Allow the end of this tube to dip in a little water in a beaker. Apply suction to the outer end of the potash bulbs till the water rises a few inches in the bent tube. Then clamp the rubber on the end of the potash bulbs. If tight, the water level in the bent tube will not drop perceptibly within half an hour. If otherwise, locate the leak by testing each section.
Check the weight of the tubes and bulbs. Transfer 0.5 gm. of the finely powdered coal, dried for half an hour at 103° C., to a weighed porcelain boat, and place in the tube as shown. See that all connections are right. Turn on a very slow stream of oxygen. Gradually heat up the part of the tube containing the copper oxide, advancing towards the boat till all the oxide is at a dull red heat, and finally the entire tube from a to d attains that temperature.
When the coal is completely oxidised (all black particles have disappeared; gradually turn off the bunsens and reduce the temperature, passing a slow stream of oxygen meanwhile. Remove the tubes and bulbs, and weigh. The following example shows how the results are entered:—
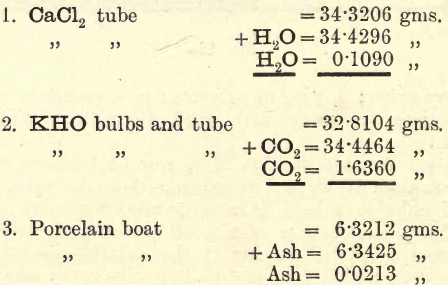
The results now are—

The nitrogen estimation (by combustion or by Kjeldahl’s process) is omitted, but in the example has been assumed, in order to show the complete calculation. Now as this analysis has been carried out on dried coal, moisture is not included. Assume that this moisture was found to be 2%, then when this item is inserted we have—

The calorific power is now ascertained as follows:—
Assume that the unit of heat “ one calorie ” is the quantity of heat required to raise the temperature of one kilo (1000 gm.) water 1° C.
The following data, have been obtained by experiment:—
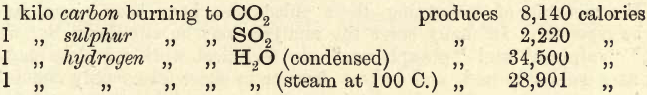
Then, if C, H, O and S represent the percentages of carbon, hydrogen, oxygen and sulphur respectively the calorific power
The student will note that in this formula there is deducted from the %H an amount sufficient to combine with all the O present to form H2O. As H and O so united in the coal are of no value for heating purposes, this quantity of H is accordingly deducted as shown.
Now, in practice, when H burns, a considerable amount of the products of combustion escape as steam and are not condensed, and in this state a certain amount of the heat becomes latent, and for heating purposes is not available. Also, whatever moisture is in the coal has to be heated up to boiling point, and converted into steam at the expense of the carbon and hydrogen in the coal. The heat so consumed is again not available in practice; hence the following modification of the formula gives more accurate results:—
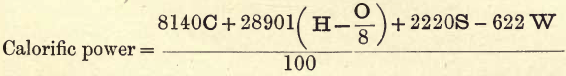
(Where W=% moisture and 622 = calories necessary to change 1 kilo water at 15° C. into steam at 100 C.).

Analysis of Cokes
The student need not, unless he have ample time, proceed with a detailed analysis of coke. A good coke should be low in ash, sulphur, and phosphorus, and high in fixed carbon. It also should be up to a certain physical standard as regards crushing resistance, porosity, specific gravity, etc. (consult Coke: its Manufacture and Recovery of Bye-Products, by John Fulton). The same authority quotes the following as a chemical standard for good coke:—
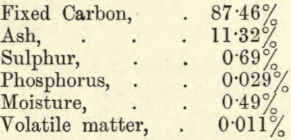
The methods of estimating these substances have been given, and need not be repeated. In many cases the analysis may be cut down to “ fixed carbon,“sulphur,” and “phosphorus.” In technical work complete analyses are not, as a rule, required, and only a few, or in some cases only one substance requires estimation. The chemist in such cases modifies the complete scheme accordingly.
